|
|
|
Kiss-1 / Metastin / GPR54 / AXOR12 |

|
OBJECTIVE: To investigate whether pregnancies with small for gestational age (SGA) neonates, defined as customized birth weight below the 10th centile, are associated with altered levels of metastin in maternal plasma in the first trimester.
STUDY DESIGN: Maternal blood was obtained between 8 and 14 weeks of pregnancy. Levels of metastin were measured in pregnancies with (n = 31) or without SGA-neonates (n = 31), matched for gestational age at venipuncture. Measurement of beta-hCG was included to study the influence of gestational age and placental volume on plasma levels of the measured markers.
RESULTS: Metastin was significantly lower in SGA-pregnancies compared to an equal number of matched uneventful pregnancies (metastin: 1376 +/- 1317 pmol/L vs 2035 +/- 1260 pmol/L, p = 0.035; mean +/- standard deviation)(Cat. # : RK-048-56). beta-hCG levels were not different.
CONCLUSION: Metastin is significantly lower in maternal plasma in the first trimester, in pregnancies with SGA-neonates. It might therefore be used in combination with other markers for risk estimation of growth impairment in the first trimester.
Smets et al. Prenat Diagn. 2008 Apr;28(4):299-303.
|
|

|
Kisspeptins (Kp), peptide products of the Kisspeptin-1 (KISS1) gene are endogenous ligands for a G protein-coupled receptor 54 (GPR54). Previous findings have shown that KISS1 acts as a metastasis suppressor in numerous cancers in humans. However, recent studies have demonstrated that an increase in KISS1 and GPR54 expression in human breast tumors correlates with higher tumor grade and metastatic potential. At present, whether or not Kp signaling promotes breast cancer cell invasiveness, required for metastasis and the underlying mechanisms, is unknown. We have found that kisspeptin-10 (Kp-10), the most potent Kp, stimulates the invasion of human breast cancer MDA-MB-231 and Hs578T cells using Matrigel-coated Transwell chamber assays and induces the formation of invasive stellate structures in three-dimensional invasion assays. Furthermore, Kp-10 stimulated an increase in matrix metalloprotease (MMP)-9 activity. We also found that Kp-10 induced the transactivation of epidermal growth factor receptor (EGFR). Knockdown of the GPCR scaffolding protein, ?arrestin 2, inhibited Kp-10-induced EGFR transactivation as well as Kp-10 induced invasion of breast cancer cells via modulation of MMP-9 secretion and activity. Finally, we found that the two receptors associate with each other under basal conditions, and FRET analysis revealed that GPR54 interacts directly with EGFR. The stability of the receptor complex formation was increased upon treatment of cells by Kp-10. Taken together, our findings suggest a novel mechanism by which Kp signaling via GPR54 stimulates breast cancer cell invasiveness.
Zajak et al. PLoS One. 2011;6(6):e21599. Epub 2011 Jun 28.
[Note: Article features Phoenix Kisspeptin-10]
|
|

|
Kisspeptins are biologically active cleavage peptides of the KiSS-1 gene products with important roles in the suppression of tumor metastasis and in the reproduction. The aim of the present study is to clarify changes of the expression of kisspeptins and kisspeptin receptor in the kidney with and without chronic renal impairment. 5/6 nephrectomized rats were used as the rat model of chronic renal failure. Competitive quantitative RT-PCR showed that kisspeptin mRNA levels were decreased in the kidney of 5/6 nephrectomized rats at 56 days compared with sham-operated rats. In contrast, immunoreactive kisspeptin concentrations were increased in the kidney of 5/6 nephrectomized rats at 56 days. On the other hand, kisspeptin receptor mRNA levels were increased in the kidney of 5/6 nephrectomized rats at 14 and 56 days compared with sham-operated rats. Immunocytochemistry showed that kisspeptins and kisspeptin receptor were expressed in renal tubular cells, collecting duct cells, vascular smooth muscle cells in both rats. The intensity of kisspeptin receptor immunostaining was lower in 5/6 nephrectomized rats than in sham-operated rats. Western blot analysis confirmed that kisspeptin receptor protein levels were significantly decreased in the remnant kidney of 5/6 nephrectomized rats (about 23% of sham-operated rats), which is a good contrast to the kisspeptin receptor mRNA expression. The present study has shown that expression of kisspeptins and kisspeptin receptor are altered in the kidney tissues of chronic renal impairment, raising the possibility of their pathophysiological roles in chronic renal failure.
Shoji et al. Peptides. 2010 Oct;31(10):1920-5. Epub 2010 Jul 17.
|
|

|
Spontaneously hypertensive (SH) rats, extensively used as experimental models of essential human hypertension, display important alterations in the neuroendocrine reproductive axis, which manifest as markedly delayed puberty onset in females but whose basis remains largely unknown. We analyze herein in female SH rats: 1) possible alterations in the expression and function of KiSS-1/GPR54 and GnRH/GnRH-receptor systems, 2) the integrity of feedback mechanisms governing the hypothalamic-pituitary-ovarian axis, and 3) the control of ovarian function by gonadotropins. Our data demonstrate that, despite overtly delayed puberty, no significant decrease in hypothalamic KiSS-1, GPR54, or GnRH mRNA levels was detected in this strain. Likewise, in vivo gonadotropin responses to ovariectomy and systemic kisspeptin-10 or GnRH administration, as well as in vitro gonadotropin responses to GnRH, were fully preserved in SH rats. Moreover, circulating LH levels were grossly conserved during prepubertal maturation, whereas FSH levels were even enhanced from d 20 postpartum onwards. In striking contrast, ovarian weight and hormone (progesterone and testosterone) responses to human chorionic gonadotropin (CG) in vitro were profoundly decreased in SH rats, with impaired follicular development and delayed ovulation at puberty. Such reduced hormonal responses to human CG could not be attributed to changes in LH/CG or FSH-receptor mRNA expression but might be linked to blunted P450scc, 3beta-hydroxy steroid dehydrogenase, and aromatase mRNA levels in ovaries from SH rats. In conclusion, our results indicate that the expression and function of KiSS-1/GPR54 and GnRH/GnRH-receptor systems is normal in SH rats, whereas ovarian development, steroidogenesis, and responsiveness to gonadotropins are strongly compromised.
PMID:
19228890
Pinilla et al. Endocrinology. 2009 Jun;150(6):2889-97. Epub 2009 Feb 19.
|
|

|
Kisspeptin (also known as metastin), a hypothalamic peptide, has attracted attention as a key molecule in the release of gonadotrophin-releasing hormone (GnRH) in various mammalian species, such as rodents, sheep and primates. Two populations of kisspeptin neurones in the brain may control two modes of GnRH release to time the onset of puberty and regulate oestrous cyclicity in rats and mice. One population of kisspeptin neurones, located in the anteroventral periventricular nucleus, appears to be responsible for the induction of the GnRH surge that leads to the luteinising hormone surge and ovulation. The other, located in the hypothalamic arcuate nucleus, appears to be involved in generating GnRH pulses, resulting in luteinising hormone pulses followed by follicular development and steroidogenesis in the ovary. The present review focuses on the physiological role of the two populations of kisspeptin neurones in controlling gonadal functions by generating the two modes of GnRH release in a female rat model.
Uenoyama et al. J Neuroendocrinol. 2009 Mar;21(4):299-304.
|
|

|
Kisspeptin, the product derived from KiSS-1, and its cognate receptor, GPR54, both exert a role in the neuroendocrine control of reproduction by regulating gonadotrophin-releasing hormone (GnRH) secretion. In the present study, we demonstrate, using dual immunofluorescence with specific antibodies, that the KiSS-1 and GPR54 genes are both expressed in rat gonadotrophs. All luteinising hormone β-immunoreactive (LHβ-ir) cells were stained by the KiSS-1 antibody but some kisspeptin-ir cells were not LHβ positive; thus, we cannot exclude the possibility that kisspeptins are expressed in other pituitary cells. All GPR54-ir are co-localised with LHβ cells, but only a subset of LHβ cells are stained with the GPR54 antibody. Using the real-time reverse transcription-polymerase chain reaction (RT-PCR), we found that the expression of KiSS-1 and GPR54 is differentially regulated by steroids. In the female, KiSS-1 mRNA levels dramatically decreased following ovariectomy (OVX), and this decrease was prevented by administration of 17β-oestradiol (E2), but not by administration of GnRH antagonist or agonist. Administration of E2 in OVX rats receiving either GnRH antagonist or agonist clearly shows that E2 acts directly on the pituitary to positively control KiSS-1 expression. In OVX rats, administration of the selective oestrogen receptor (ER)α ligand propylpyrazoletriol, but not the selective ERβ ligand diarylpropionitrile, mimics this effect. By contrast, our study shows that GPR54 expression is positively regulated by GnRH and negatively controlled by chronic exposure to E2. In summary, our data document for the first time that, in the female rat pituitary, KiSS-1 expression is up-regulated by oestradiol, similarly to that seen in the anteroventral periventricular nucleus of the hypothalamus. Conversely, GPR54 is up-regulated by GnRH, which exclusively targets gonadotrophs.
Richard et al. J Neuroendocrinol. 2008 Mar;20(3):381-93. Epub 2008 Jan 17.
|
|

|
The gonadotropic axis is centrally controlled by a complex regulatory network of excitatory and inhibitory signals that is activated at puberty. Recently, loss-of-function mutations of the gene encoding GPR54, the putative receptor for the KiSS-1 derived peptide metastin, have been associated to lack of puberty onset and hypogonadotropic hypogonadism. Yet, the pattern of expression and functional role of the KiSS-1/GPR54 system in the rat hypothalamus remain so far unexplored. In the present work, expression analyses of KiSS-1 and GPR54 genes were conducted in different physiological and experimental settings, and the effects of central administration of KiSS-1 peptide upon LH release were assessed in vivo. Persistent expression of KiSS-1 and GPR54 mRNAs was detected in rat hypothalamus throughout postanatal development, with maximum expression levels at puberty both in male and female rats. Hypothalamic expression of KiSS-1 and GPR54 genes changed along the estrous cycle and was significantly increased after gonadectomy; a rise that was prevented by sex steroid replacement both in males and females. Moreover, hypothalamic expression of KiSS-1 gene was sensitive to neonatal imprinting by estrogen. From a functional standpoint, intracerebroventricular administration of KiSS-1 peptide induced a dramatic increase in serum LH levels in prepubertal male and female rats, as well as in adult animals. In conclusion, we provide novel evidence of the develpmental and hormonally regulated expression of KiSS-1 and GPR54 mRNAs in rat hypothalamus, and on the ability of KiSS-1 peptide to potently stimulate LH secretion in vivo. Overall, our current data support the contention that the hypothalamic KiSS-1/GPR54 system is a pivotal factor in the central regulation of the gonadotropic axis at puberty and adulthood.
Navarro et al. Endocrinology. 2004 Oct;145(10):4565-74. Epub 2004 Jul 8.
|
|

|
Kisspeptins are products of the KiSS-1 gene, which bind to a G protein-coupled receptor known as GPR54. Mutations or targeted disruptions in the GPR54 gene cause hypogonadotropic hypogonadism in humans and mice, suggesting that kisspeptin signaling may be important for the regulation of gonadotropin secretion. To examine the effects of kisspeptin-54 (metastin) and kisspeptin-10 (the biologically active C-terminal decapeptide) on gonadotropin secretion in the mouse, we administered the kisspeptins directly into the lateral cerebral ventricle of the brain and demonstrated that both peptides stimulate LH secretion. Further characterization of kisspeptin-54 demonstrated that it stimulated both LH and FSH secretion, at doses as low as 1 fmol; moreover, this effect was shown to be blocked by pretreatment with acyline, a potent GnRH antagonist. To learn more about the functional anatomy of kisspeptins, we mapped the distribution of KiSS-1 mRNA in the hypothalamus. We observed that KiSS-1 mRNA is expressed in areas of the hypothalamus implicated in the neuroendocrine regulation of gonadotropin secretion, including the anteroventral periventricular nucleus, the periventricular nucleus, and the arcuate nucleus. We conclude that kisspeptin-GPR54 signaling may be part of the hypothalamic circuitry that governs the hypothalamic secretion of GnRH.
Gottsch et al. Endocrinology. 2004 Sep;145(9):4073-7. Epub 2004 Jun 24
|
|

|
BACKGROUND: Puberty, a complex biologic process involving sexual development, accelerated linear growth, and adrenal maturation, is initiated when gonadotropin-releasing hormone begins to be secreted by the hypothalamus. We conducted studies in humans and mice to identify the genetic factors that determine the onset of puberty.
METHODS: We used complementary genetic approaches in humans and in mice. A consanguineous family with members who lacked pubertal development (idiopathic hypogonadotropic hypogonadism) was examined for mutations in a candidate gene, GPR54, which encodes a G protein-coupled receptor. Functional differences between wild-type and mutant GPR54 were examined in vitro. In parallel, a Gpr54-deficient mouse model was created and phenotyped. Responsiveness to exogenous gonadotropin-releasing hormone was assessed in both the humans and the mice.
RESULTS: Affected patients in the index pedigree were homozygous for an L148S mutation in GPR54, and an unrelated proband with idiopathic hypogonadotropic hypogonadism was determined to have two separate mutations, R331X and X399R. The in vitro transfection of COS-7 cells with mutant constructs demonstrated a significantly decreased accumulation of inositol phosphate. The patient carrying the compound heterozygous mutations (R331X and X399R) had attenuated secretion of endogenous gonadotropin-releasing hormone and a left-shifted dose-response curve for gonadotropin-releasing hormone as compared with six patients who had idiopathic hypogonadotropic hypogonadism without GPR54 mutations. The Gpr54-deficient mice had isolated hypogonadotropic hypogonadism (small testes in male mice and a delay in vaginal opening and an absence of follicular maturation in female mice), but they showed responsiveness to both exogenous gonadotropins and gonadotropin-releasing hormone and had normal levels of gonadotropin-releasing hormone in the hypothalamus.
CONCLUSIONS: Mutations in GPR54, a G protein-coupled receptor gene, cause autosomal recessive idiopathic hypogonadotropic hypogonadism in humans and mice, suggesting that this receptor is essential for normal gonadotropin-releasing hormone physiology and for puberty.
Seminara et al. N Engl J Med. 2003 Oct 23;349(17):1614-27.
|
|

|
Metastin is a novel peptide that was recently isolated from human placenta as the endogenous ligand of an orphan heptahelical receptor, hOT7T175. Metastin has been shown to suppress the motility of hOT7T175-transfected melanoma cells; however, studies of the physiological function of metastin have begun only recently. To investigate the possibility that metastin is an endocrine peptide, we determined the immunoreactive (ir-) metastin concentration in human plasma using our newly developed, sensitive, and specific two-site enzyme immunoassay. The plasma concentrations of ir-metastin in males and females were 1.30 0.14 (n = 12) and 1.31 0.37 fmol/ml (n = 10), respectively. As metastin is known to be abundant in human placenta, the ir-metastin concentration in the maternal plasma was then determined. The ir-metastin concentrations were 1230 346 fmol/ml (n = 11) in the first trimester, 4590 555 (n = 16) in the second trimester, and 9590 1640 (n = 12) in the third trimester. On d 5 after delivery, the ir-metastin concentration returned to nearly the nonpregnant level (7.63 1.33 fmol/ml; n = 10), suggesting that ir-metastin increases in pregnancy and is derived mainly from the placenta. The plasma from both nonpregnant and pregnant women showed a single ir-metastin peak at the same retention time as authentic metastin on reverse phase HPLC analysis, indicating that the major portion of the circulating metastin, as determined by our two-site enzyme immunoassay, represents endogenous metastin. Histochemical studies of human placenta localized metastin mRNA and immunoreactivity to the syncytiotrophoblasts. The present study provides evidence for metastin as a novel placenta-derived hormone in humans.
Horikoshi et al. J Clin Endocrinol Metab 2003 Feb;88(2):914-9
|
|

|
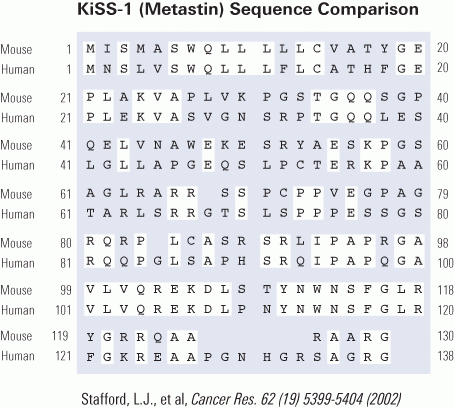
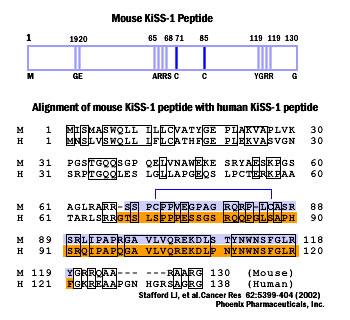
G-protein-coupled receptors receive many different signals to activate different functions such as cellgrowth, proliferation, and migration. KiSS1 is a metastasis suppressor gene that has been shown to inhibit metastasis of human melanomas and breast carcinomas. The human KiSS1 gene encodes a COOH-terminally amidated active peptide, and this peptide is the ligand of a novel G-protein-coupled receptor. However, the mechanism of the antimetastatic actions of KiSS1 and its G-protein-coupled receptor has not been elucidated. In this study, we identified the mouse homologues of the KiSS1 peptide and its G-protein-coupled receptor and characterized the signaling pathways mediated by the activation of the KiSS1 receptor. Although human and mouse KiSS1 proteins share relatively low overall homology (52%), the active peptides (10-amino-acid residues) are highly conserved between mouse and human KiSS1 proteins, varying by only one conserved amino acid [Tyr (Y) to Phe (F)]. Activation of the receptor by KiSS1 peptide leads to the activation of G-protein-activated phospholipase C (PLC-beta), which suggests direct coupling of the KiSS1 peptide to the Galphaq-mediate PLC-Ca2+ signaling pathway. Furthermore, activation of the KiSS1 receptor inhibits cell proliferation and cell migration, key characteristics of tumor metastasis.
Stafford et al. Cancer Res 2002 Oct 1;62(19):5399-404
|
|

|
Metastasis is a major cause of death in cancer patients and involves a multistep process including detachment of cancer cells from a primary cancer, invasion of surrounding tissue, spread through circulation, re-invasion and proliferation in distant organs. KiSS-1 is a human metastasis suppressor gene, that suppresses metastases of human melanomas and breast carcinomas without affecting tumorigenicity. However, its gene product and functional mechanisms have not been elucidated. Here we show that KiSS-1 (refs 1, 4) encodes a carboxy-terminally amidated peptide with 54 amino-acid residues, which we have isolated from human placenta as the endogenous ligand of an orphan G-protein-coupled receptor (hOT7T175) and have named 'metastin'. Metastin inhibits chemotaxis and invasion of hOT7T175-transfected CHO cells in vitro and attenuates pulmonary metastasis of hOT7T175-transfected B16-BL6 melanomas in vivo. The results suggest possible mechanisms of action for KiSS-1 and a potential new therapeutic approach.
Ohtaki et al. Nature 2001 May 31;411(6837):613-7
|
|

|
Metastin, the product of metastasis suppressor gene KiSS-1, is proposed to be the natural ligand for the G-protein-coupled receptor GPR54, known also as AXOR12. This immunohistochemical study, using a rabbit polyclonal antiserum against the human metastin fragment (45-54)-NH(2)/Kisspeptin-10 (Catalog No.: H-048-56), showed that in rats metastin-like immunoreactivity (MTS-LI) was present in neurons of the nucleus of the solitary tract and caudoventrolateral reticular nucleus, and in cell processes of the spinal trigeminal tract and lateral reticular nucleus. MTS-LI was confined mainly to neurons and fibers at or caudal to the area postrema. In the spinal cord, MTS-LI cell processes formed a dense plexus in superficial layers I and II of the dorsal horn. The pattern of distribution of MTS-LI in the medulla and spinal cord suggests that this novel peptide may participate in autonomic and sensory neural signaling.
Dun et al. Neurosci Lett 2003 Jan 2;335(3):197-201
|
|
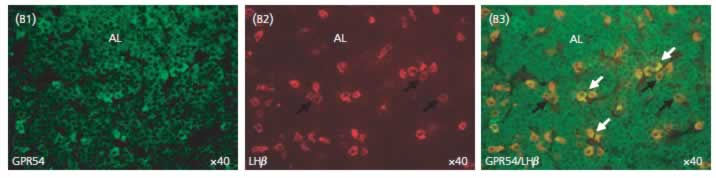
Fluorescent immunohistochemistry
was carried out using fluorescein for GPR54 (green) and Texas Red for LHb (red). Dual-labelled cells stand out in yellow ⁄ orange when images
are merged (B3). The rare staining for GPR54 was found to be restricted to the anterior lobe (AL, A1). The white arrows show positive cells for GPR54 which are
positive for LHb (B3) and the dark arrows display some LHb cells which are not stained by GPR54 antibody (B2, B3). These immunofluorescent data shows that
GPR54 is expressed in gonadotroph cells in the rat. No specific staining was detected in the absence of primary antibody (control-).
Richard et al. J Neuroendocrinol. 2008 Mar;20(3):381-93. Epub 2008 Jan 17.
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
No primary antibody |
Pretreatment |
N/A |
Blocking |
2% Normal Goat Serum |
Primary Antibody |
Rabbit Anti-GRP54 (375-396) (Mouse) Serum (Catalog No.:H-001-64) |
Optimal Dilution |
1:50-100 (1hour at RT) |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification |
ABC (Vector) (1:400, 30 min) |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |
AXOR12 (OT7T175, GPR54) is a newly identified orphan G Protein-Coupled Receptor for KiSS-1/Metastin. Mouse pancreas and placenta as well as human placenta tissues can be stained with Phoenix Rabbit anti-AXOR12 (375-398) (Human) antibody (H-048-61).
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
No primary antibody |
Pretreatment |
Intact; Target Retrieval 25 min (Steam) |
Blocking |
2% Normal Goat Serum |
Primary Antibody |
Anti-AXOR12 (375-398) (Human) serum (H-048-61) |
Optimal Dilution |
1: 100 |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification |
ABC (Vector) |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |

Sections of human FT placental tissue at week 6-10 of gestation showing the localization of KiSS-1/Kp-54 (A,B) and KiSS-1R (E,F). KiSS-1 and KiSS-1R mRNA were detected by in situ hybridization as dark blue precipitates, whereas their respective proteins were detected using affinity purified polyclonal antibodies evident as dark red precipitate. Sense probes (C,G) and nonimmune sera (D,H) produced no detectable signal. KiSS-1 mRNA (A) and Kp-145/Kp-54 protein (B) were detected mainly on the outer (syncytiotrophoblast) surface of villi. Higher magnification showed that KiSS-1/Kp-54 expression is restricted to the syncytiotrophoblast (insets; A and B; bars, 25 µm), whereas it was undetectable in villous (vCT) and extravillous (evCT) cytotrophoblasts. KiSS-1R mRNA is located in the syncytiotrophoblast (ST), villous (vCT) and extravillous (evCT) cytotrophoblasts (E). This mRNA staining pattern is paralleled by that of KiSS-1R protein (F). Bars, 50 µm.
Bilban et al. J Cell Sci. 2004 Mar 15;117(Pt 8):1319-28.
Fixative |
4%paraformaldehyde/ 4%picric acid |
Embedding |
Paraffin |
Negative Control |
No primary antibody or primary antibody pre-absorbed with the metastin (45-54)-NH2 peptide (10µg/ml) overnight |
Pretreatment |
Intact |
Blocking |
2% Normal Goat Serum |
Primary Antibody |
Metastin (45-54) (Human) Antibody (Code:H-048-56) |
Optimal Dilution |
1: 1500 |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:150) |
Amplification |
ABC (Vector) |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
No |
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
No primary antibody |
Pretreatment |
Intact |
Blocking |
2% Normal Goat Serum |
Primary Antibody |
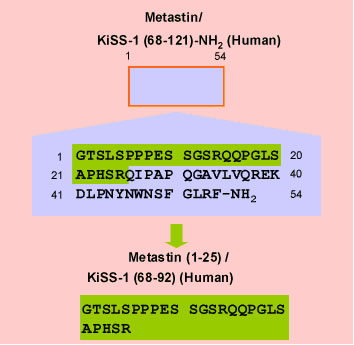
Rabbit Anti-Metastin (1-25) / KiSS-1 (68-92) (Human) Antiserum (Catalog No.:H-048-62) |
Optimal Dilution |
1:500 (overnight at 4 C) |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification |
ABC (Vector) |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |


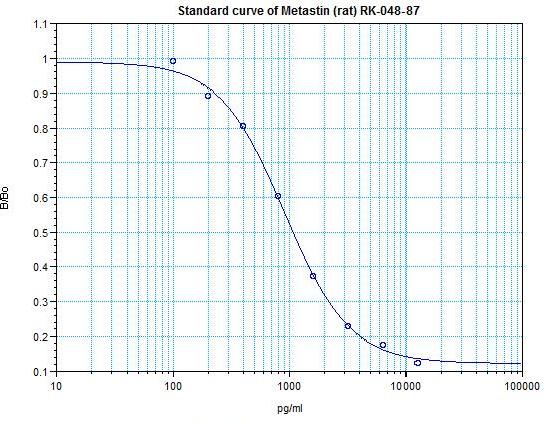
Metastin (Rat) / Kiss-1 (69-119)-amide
|
100 |
| Kiss-1 (68-119)-Amide (Mouse) |
60 |
| Metastin (26-54) [pGlu26] Amide (Human)/Kiss-1[pGlu26} (93-121)-Amide |
0 |
| Metastin (1-54)-Amide (Human) / Kiss-1 (68-121)-Amide |
0 |
| Metastin (45-54) (Human) / Kiss-1 (112-119)-Amide |
0 |
| Prepro-Kiss-1 (20-65) (Mouse) |
0 |
Identities are indicated, and putative transmembrane domains are boxed. Putative N-glycosylation sites are underlined, and potential phosphorylation sites by PKC are indicated as bold characters.


 
 
|
|
|
metastin-small-agonist;Kisspeptin_Antagonist
%001-64%;%048-61%;%metastin%
|
|
|


