Adipose tissue secretes proteins referred to as adipokines, many of which promote inflammation and disrupt glucose homeostasis. Here we show that secreted frizzled-related protein 5 (Sfrp5), a protein previously linked to the Wnt signaling pathway, is an anti-inflammatory adipokine whose expression is perturbed in models of obesity and type 2 diabetes. Sfrp5-deficient mice fed a high-calorie diet developed severe glucose intolerance and hepatic steatosis, and their adipose tissue showed an accumulation of activated macrophages that was associated with activation of the c-Jun N-terminal kinase signaling pathway. Adenovirus-mediated delivery of Sfrp5 to mouse models of obesity ameliorated glucose intolerance and hepatic steatosis. Thus, in the setting of obesity, Sfrp5 secretion by adipocytes exerts salutary effects on metabolic dysfunction by controlling inflammatory cells within adipose tissue.
Ouchi et al.
Science. 2010 Jul 23;329(5990):454-7. Epub 2010 Jun 17
1. decrease in Sfrp5 production
2. increase the Wnt5a activation of JNK1 to impair IRS-1 activity and insulin signaling
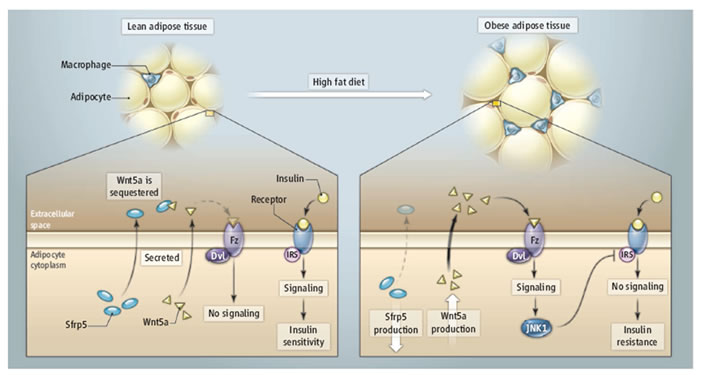
Sfrp5 is secreted from adipocytes and sequesters Wnt5a, preventing noncanonical pathway activation. In the obesity state, adipocytes enlarge and secrete more Wnt5a but less Sfrp5. Macrophages present in the obese adipose tissue also respond to Wnt5a and produce proinflammatory cytokines. Such results lead to insulin resistance.
Oh et al. Science. 2010 Jul 23;329(5990):397-8.
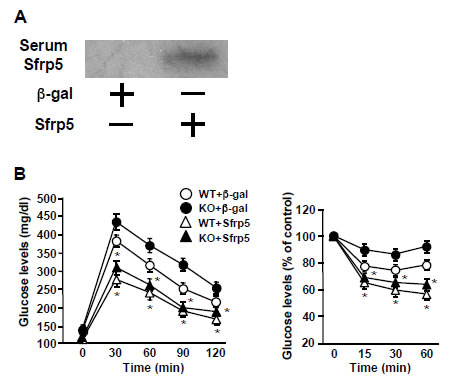
Fig S10: After 10 weeks of HF/HS diet feeding, WT and KO mice were intravenously treated with
AdTRE-β-gal (2.5x108 pfu total) or AdTRE-Sfrp5 (2.5x108 pfu total) along with AdCMV-tTA
(2.5x108 pfu total). (A) Detection of Sfrp5 in serum of WT mice following adenovirus-mediated intravenous injection of Sfrp5. At 1 week after treatment of WT mice with adenoviral vectors (β-gal or Sfrp5), serum was collected. Sfrp5 protein level in serum (10 μl) was determined by immunoblot analysis. (B) Glucose tolerance test (left) and insulin tolerance test (right) at 2
weeks after injection of adenoviral vectors (β-gal or Sfrp5) (mean ± SEM, n=6-7 in each
group). *, P<0.01 vs. corresponding β-gal treatment.
Ouchi et al.
Science. 2010 Jul 23;329(5990):454-7. Epub 2010 Jun 17
Mitochondria serve a critical role in physiology and disease. The genetic basis of mitochondrial regulation in mammalian cells has not yet been detailed. We performed a large-scale RNAi screen to systematically identify genes that affect mitochondrial abundance and function. This screen revealed previously unrecognized roles for >150 proteins in mitochondrial regulation. We report that increased Wnt signals are a potent activator of mitochondrial biogenesis and reactive oxygen species (ROS) generation, leading to DNA damage and acceleration of cellular senescence in primary cells. The signaling protein insulin receptor substrate-1 (IRS-1), shown here to be a transcriptional target of Wnt, is induced in this setting. The increased level of IRS-1 drives activation of mitochondrial biogenesis; furthermore, in insulin-responsive cell types, it enhances insulin signaling, raising the possibility that Wnt proteins may be used to modulate glucose homeostasis. Our results identify a key component of the mitochondrial regulatory apparatus with a potentially important link to metabolic and degenerative disorders.
Yoon et al. Genes Dev. 2010 Jul 15;24(14):1507-18.
High phenotypic variation in diet-induced obesity in male C57BL/6J inbred mice suggests a molecular model to investigate non-genetic mechanisms of obesity. Feeding mice a high-fat diet beginning at 8 wk of age resulted in a 4-fold difference in adiposity. The phenotypes of mice characteristic of high or low gainers were evident by 6 wk of age, when mice were still on a low-fat diet; they were amplified after being switched to the high-fat diet and persisted even after the obesogenic protocol was interrupted with a calorically restricted, low-fat chow diet. Accordingly, susceptibility to diet-induced obesity in genetically identical mice is a stable phenotype that can be detected in mice shortly after weaning. Chronologically, differences in adiposity preceded those of feeding efficiency and food intake, suggesting that observed difference in leptin secretion is a factor in determining phenotypes related to food intake. Gene expression analyses of adipose tissue and hypothalamus from mice with low and high weight gain, by microarray and qRT-PCR, showed major changes in the expression of genes of Wnt signaling and tissue re-modeling in adipose tissue. In particular, elevated expression of SFRP5, an inhibitor of Wnt signaling, the imprinted gene MEST and BMP3 may be causally linked to fat mass expansion, since differences in gene expression observed in biopsies of epididymal fat at 7 wk of age (before the high-fat diet) correlated with adiposity after 8 wk on a high-fat diet. We propose that C57BL/6J mice have the phenotypic characteristics suitable for a model to investigate epigenetic mechanisms within adipose tissue that underlie diet-induced obesity.
Koza et al. PLoS Genet. 2006 May;2(5):e81. Epub 2006 May 26.
WNT binding domain with or without Fc fusion. SFRP5 sequesters WNT proteins in the extracellular space and prevents WNT binding to its frizzled receptor. Obesity leads to higher levels of WNT-5a expression and increases in the ratio of WNT-5a to SFRP5. The WNT binding protein SFRP5 fuses to the Fc fragment of human IgG1. This fusion renders stability of the protein in vitro and in vivo without significant loss of activity.
WNT-5a triggers the so-called noncanonical WNT signaling which suppresses the β-catenin pathway and activates the planar cell polarity pathway, respectively. Like the canonical pathway, the noncanonical WNT signaling is one of the most important signaling pathways in animal development and stem cell function. It has been shown that the noncanonical WNT pathway activated by WNT-5a is crucial in cardiac development and derivation of dopaminergic neurons from embryonic stem cells.Phoenix Pharmaceuticals, Inc. is currently the sole commercial source of bioactive human WNT-5a which is extremely difficult to produce and purify by conventional methodologies. We have developed a novel and proprietary process to express and purify WNT-5a from a human cell expression system in Xeno-free medium.
The Frizzled-5 is fused to the Fc fragment of human IgG1. This fusion renders stability of the protein in vitro and in vivo without significant loss of activity. Frizzled-5 Fc fusion binds to WNT-5a in a way similar as SFRP5.
The Frizzled-8 is fused to the Fc fragment of human IgG1. This fusion renders stability of the protein in vitro and in vivo without significant loss of activity. Frizzled-8 Fc fusion binds to WNT-5a in a way similar as SFRP5.
R-spondin-1 is a natural enhancer of the canonical Wnt pathway. When used together with Wnt proteins that activate the beta-catenin pathway, Rspondin-1 enhances the activity of Wnt


