|
|
|
Visfatin / PBEF |
A New Natural Insulin-mimetic Adipokine |
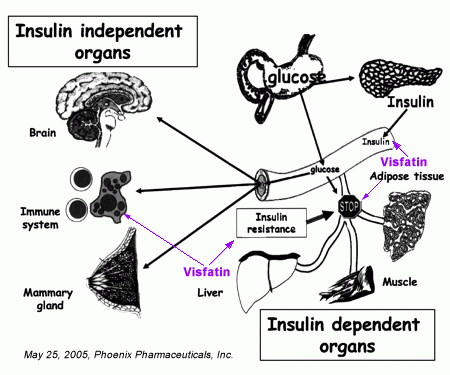
Visfatin is a newly discovered adipocyte hormone with a direct relationship between plasma visfatin level and type 2 diabetes mellitus. Visfatin binds to the insulin receptor at a site distinct from that of insulin and causes hypoglycaemia by reducing glucose release from liver cells and stimulating glucose utilization in adipocytes and myocytes. Visfatin is upregulated by hypoxia, inflammation and hyperglycaemia and downregulated by insulin, somatostatin and statins.
This hormone is found in the cytoplasm as well as the nucleus of cells and has been identified in many tissues and organs including the brain, kidney, lung, spleen and testis but preferentially expressed in visceral adipose tissue and upregulated in some animal models of obesity. Visceral adipose tissue is regarded to be more pernicious than subcutaneous adipose tissue. Visfatin is an endocrine, autocrine as well as paracrine peptide with many functions including enhancement of cell proliferation, biosynthesis of nicotinamide mono- and dinucleotide and hypoglycaemic effect.
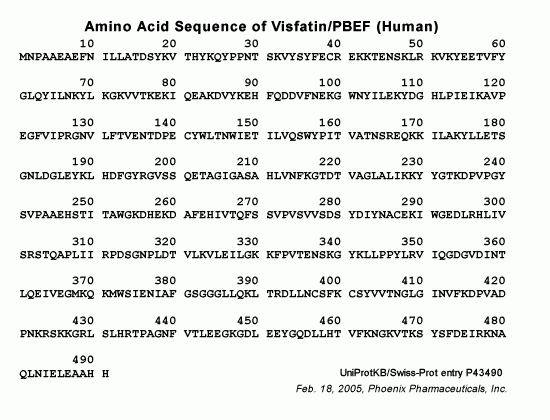
Visfatin, also known as a pre-B cell colony-enhancing factor, consists of 491 amino acids (aa) in human, chimpanzee, cattle, pig, rat and mouse, 490 aa in rhesus monkey, 285 aa in sheep, 587 in opossum and 588 aa in canines. Visfatin gene is well preserved during evolution. For example, the canine visfatin protein sequence is 96% and 94% identical to human and rodent visfatin, respectively. Since evidence of a direct link between visfatin genotype and human type 2 diabetes mellitus is still weak, more molecular, physiological and clinical studies are needed to determine the role of visfatin in the etiology and pathogenesis of type 2 diabetes mellitus.
Adeghate E., Curr Med Chem. 2008;15(18):1851-62.

Murphy et al. Nat Med. 2006 Jan;12(1):32-3.
Fat tissue produces a variety of secreted proteins (adipocytokines) with important roles in
metabolism. We isolated a newly identified adipocytokine, visfatin, that is
highly enriched in the visceral fat of both humans and mice and whose expression
level in plasma increases during the development of obesity. Visfatin
corresponds to a protein identified previously as pre-B cell colony-enhancing
factor (PBEF), a 52-kilodalton cytokine expressed in lymphocytes. Visfatin
exerted insulin-mimetic effects in cultured cells and lowered plasma glucose
levels in mice. Mice heterozygous for a targeted mutation in the visfatin gene
had modestly higher levels of plasma glucose relative to wild-type littermates.
Surprisingly, visfatin binds to and activates the insulin receptor. Further
study of visfatin's physiological role may lead to new insights into glucose
homeostasis and/or new therapies for metabolic disorders such as
diabetes.
Fukuhara et al. Science. 2005 Jan 21;307(5708):426-30.

Cherrington. J Clin Invest. 2005 May;115(5):1136-9.


Model depicting the control of energy homeostasis and hepatic glucose metabolism by
adiposity- and nutrient-related signals. Neuronal systems sense and respond to
input from hormones such as insulin and leptin that are secreted in proportion
to body energy stores and from the metabolism of circulating nutrients (such as
glucose and FFAs). In response to this input, adaptive changes occur in energy
intake, energy expenditure, and hepatic glucose production.
Schwartz & Porte. Science. 2005 Jan 21;307(5708):375-9.

Havel. Diabetes. 2004 Feb;53 Suppl 1:S143-51.


Insulin binding to the extracellular domain of the insulin receptor
elicits a conformational change, which in turn leads to receptor
autophosphorylation (P) and tyrosine phosphorylation of intracellular protein
substrates. Two main branching pathways are activated by insulin: (a) One is the
MAPK signaling cascade, in which the Grb2/Sos pathway leads to activation of Ras
signaling, affecting cell proliferation and apoptosis. In view of their
mitogenic nature, these can be characterized as "growth signal" effects. (b) The
other is the IRS pathway, which leads to activation of kinases dependent upon
the heterodimeric (p85/p110) PI3K, such as Akt, also referred to as protein
kinase B (PKB); Akt modulates enzyme activities that, besides affecting NO
generation and apoptosis, control glucose, lipid, and protein metabolism. This
PI3K-branching pathway is termed the "metabolic signal." PI(4, 5)P2,
phosphoinositide 4,5 di-phosphate; PI(3, 4, 5)P3, phosphoinositide 3,4,5
tri-phosphate; PDK1 phosphoinositide–dependent kinase–1; MEK, MAPK kinase.

Venn diagram modeling the effect of the interaction between glucose toxicity and lack
of insulin on the vulnerable state of critical illness. Complications of type 1
and type 2 diabetes are explained by hyperglycemia and/or lack of insulin
effect. Critical illness is also characterized by hyperglycemia and lack of
insulin effect, but additional risk factors render both of these effects more
acutely toxic, as indicated by the blue shading. These risk factors include the
post-hypoxia reperfused state, iNOS-activated NO generation, increased
expression of GLUT-1 and GLUT-3 transporters, and cytokine-, neurological-, and
hormone-induced alterations in cellular processes. Hence, improved outcome of
critical illness with insulin-titrated maintenance of normoglycemia is likely to
be explained by the prevention of both direct glucose toxicity and
insulin-induced effects that are independent of glucose control.
Van den Berghe. J Clin Invest. 2004 Nov;114(9):1187-95.
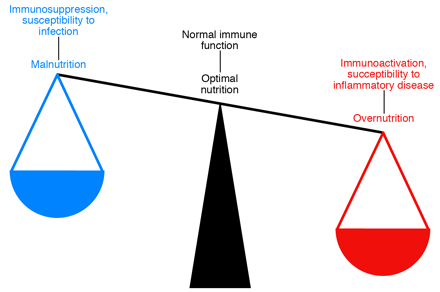
Metabolism and immunity are closely linked. Both overnutrition and undernutrition have implications for
immune function. Starvation and malnutrition can suppress immune function and
increase susceptibility to infections. Obesity is associated with a state of
aberrant immune activity and increasing risk for associated inflammatory
diseases, including atherosclerosis, diabetes, airway inflammation, and fatty
liver disease. Thus, optimal nutritional and metabolic homeostasis is an
important part of appropriate immune function and good health

Nutrient and pathogen sensing or response systems have important overlapping features, and their modulation by
obesity or infection can lead to overlapping physiological outcomes. For
example, the chronic inflammation of obesity leads to elevated plasma lipid
levels and the development of insulin resistance, eventually resulting in fatty
liver disease, atherosclerosis, and diabetes. Infection typically leads to a
more transient and robust inflammatory response and short-term hyperlipidemia
that aids in the resolution of the infection. In some circumstances of chronic
infection, however, insulin resistance, diabetes, and atherosclerosis can
result.

Wellen & Hotamisligil. J Clin Invest. 2005 May;115(5):1111-9.

(a) Normally, the
occupied insulin receptor phosphorylates scaffold proteins, such as the IRS-1,
on critical tyrosine residues. However, in insulin-resistant states, a number of
agents, such as the cytokine TNF- or circulating FFAs, lead through intermediary
signaling pathways to the activation of IKK, which in turn indirectly increases
the number of phosphorylated serine and threonine residues (indicated by blue
circles) on IRS-1. This modification blocks the tyrosine phosphorylation and
converts IRS-1 into an insulin receptor inhibitory protein. (b) In the presence
of salicylates, IKK activity is inhibited, reducing the IRS-1 serine/threonine
phosphorylation and allowing IRS-1 to be phosphorylated on tyrosine. These
phosphorylated tyrosine residues (black squares) serve as binding sites for a
number of signaling molecules, most importantly PI 3'-kinase, which initiate
signaling pathways regulating metabolism. Aspirin (ASA) also inhibits
cyclooxygensases (COX) to prevent the generation of inflammatory prostaglandins
(PGE) from arachidonic acid (AA) in a pathway unrelated to the effects of the
drug on insulin action.
Birnbaum et al. J Clin Invest. 2001 Sep;108(5):655-9.
|
|
|
| Leptin |
Improvement |
None |
| Adiponectin |
Improvement |
None |
| Adipsin / ASP |
Decline |
None |
| Resistin |
Decline |
None (rodent) |
| Resistin |
Decline |
macrophage (Human) |
| TNF-α |
Decline |
macrophage |
| IL-6 |
Decline |
macrophage |
| MCP-1 |
Decline |
macrophage |
| Visfatin |
Improvement |
Liver, lymphocytes |
| PAI-1 |
Decline |
Liver |
| Angiotensinogen |
Decline |
Liver |
| Serum amyloid A |
Not known |
Liver |
| α1-acid glycoprotein |
Not known |
Liver |
Lazar. Science. 2005 Jan 21;307(5708):373-5.
Visceral and subcutaneous adipose tissue display important metabolic differences that underlie the
association of visceral obesity with obesity-related cardiovascular and
metabolic alterations. Recently, visfatin was identified as an adipokine, which
is predominantly secreted from visceral adipose tissue both in humans and mice.
In this study, we examined whether visfatin plasma concentrations (using enzyme
immunosorbent assay, Visfatin, C-terminal, (Human) EIA Kit, catalog No.: EK-003-80, Phoenix Pharmaceuticals, Belmont,
USA) and mRNA expression (using RT-PCR) in visceral and subcutaneous
fat correlates with anthropometric and metabolic parameters in 189 subjects with
a wide range of obesity, body fat distribution, insulin sensitivity, and glucose
tolerance. Visfatin plasma concentration correlates positively with the visceral
visfatin mRNA expression (r2 = 0.17, P < 0.0001), BMI (r2 = 0.062, P =
0.004), percent body fat (r2 = 0.048, P = 0.01), and negatively with
subcutaneous visfatin mRNA expression (r2 = 0.18, P < 0.0001). However, in a
subgroup of 73 individuals, in which visceral fat mass was calculated from
computed tomography scans, there was no correlation between plasma visfatin
concentrations and visceral fat mass. We found no significant correlation
between visfatin plasma concentrations and parameters of insulin sensitivity,
including fasting insulin, fasting plasma glucose concentrations, and the
glucose infusion rate during the steady state of an euglycemic-hyperinsulinemic
clamp independent of percent body fat. Visfatin gene expression was not
different between visceral and subcutaneous adipose tissue in the entire study
group nor in selected subgroups. We found a significant correlation between
visceral visfatin gene expression and BMI (r2 = 0.06, P = 0.001) and percent
body fat (measured using dual-energy X-ray absorptiometry) (r2 = 0.044, P =
0.004), whereas no significant association between BMI or percent body fat and
subcutaneous visfatin mRNA expression existed (both P >0.5). In conclusion,
visfatin plasma concentrations and visceral visfatin mRNA expression correlated
with measures of obesity but not with visceral fat mass or waist-to-hip ratio.
In addition, we did not find differences in visfatin mRNA expression between
visceral and subcutaneous adipose tissue in humans.

Correlation between plasma visfatin concentrations and % body fat in men (A) and women (B). Data were log transformed to achieve normal distribution.
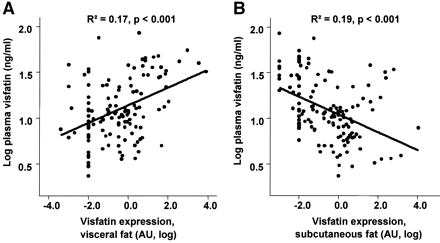
Correlation between plasma visfatin concentration and visfatin mRNA expression in visceral (A) or subcutaneous (B) fat depots (n = 163). Data were log transformed to achieve normal distribution.
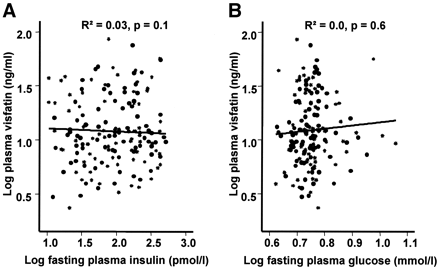
Correlation between plasma visfatin concentration, fasting plasma insulin concentrations (A), and fasting plasma glucose concentrations (B) (n = 163). Data were log transformed to achieve normal distribution.
Berndt et al. Diabetes. 2005 Oct;54(10):2911-6.
CONTEXT: The insulin-mimetic adipocytokine visfatin has been linked to obesity. The influence of weight loss
on plasma visfatin concentrations in obese subjects is unknown yet.
OBJECTIVES: In this study we investigated whether plasma visfatin concentrations are altered
by weight loss in patients with obesity.
DESIGN AND PATIENTS: In a prospective
study, fasting plasma visfatin, leptin, and adiponectin concentrations were
measured before and 6 months after gastric banding in 31 morbidly obese patients
aged 40 +/- 11 yr with a body mass index (BMI) of 46 +/- 5 kg/m(2). Fourteen
healthy subjects aged 29 +/- 5 yr with a BMI less than 25 kg/m(2) served as
controls.
RESULTS: Visfatin plasma concentrations were markedly elevated in
obese subjects (0.037 +/- 0.008 microg/ml), compared with controls (0.001 +/-
0.000 microg/ml, P < 0.001). Gastric banding reduced BMI to 40 +/- 5 kg/m(2),
visfatin to 19.2 +/- 10.9 ng/ml, and leptin from 39.0 +/- 12.4 to 29.7 +/- 10.0
ng/ml and increased adiponectin from 0.015 +/- 0.007 to 0.017 +/- 0.007
microg/ml (all P < 0.05) after 6 months. Insulin sensitivity as estimated by
the homeostasis model assessment insulin resistance index was unchanged from 5.8
+/- 3.1 to 4.6 +/- 1.9 (P = 0.13), but individual changes of insulin resistance
and visfatin were significantly associated (P < 0.05, r = -0.43).
CONCLUSIONS: Elevated plasma visfatin concentrations in morbidly obese subjects
are reduced after weight loss. This may be related to changes in insulin
resistance over time.
Haider et al. J Clin Endocrinol Metab. 2006 Apr;91(4):1578-81.
The recently discovered adipocytokine visfatin has insulin-like properties. It
lowers blood glucose and improves insulin sensitivity; however, clinical data on
visfatin are limited. To evaluate the role of visfatin in GDM (gestational
diabetes mellitus), we determined visfatin levels in women with GDM and in
healthy pregnant controls. Furthermore, visfatin concentrations were
investigated longitudinally during pregnancy and after delivery in a subgroup of
women with GDM. Blood for measurement of visfatin and metabolic parameters was
obtained from 64 women with GDM [median week of gestation, 34 (interquartile
range, 27-36) weeks] and 30 healthy pregnant controls [median week of gestation,
34 (interquartile range, 28-36) weeks]. In a subgroup of 24 women with GDM,
visfatin, leptin and metabolic parameters were investigated twice during
pregnancy (28-30 and 38-40 weeks of gestation) and 2 weeks after delivery. In
the cross-sectional analysis, median visfatin levels were significantly elevated
in women with GDM [64.0 (interquartile range, 50.9-74.8) ng/ml] compared with
controls [46.0 (interquartile range, 36.9-54.6) ng/ml; P<0.0001]. In women
with GDM, visfatin correlated with week of gestation at the time of blood draw
(R=0.35, P=0.005). No association with fasting glucose, insulin, homoeostasis
model assessment-insulin resistance or body mass index was observed. According
to the longitudinal analysis, visfatin increased during pregnancy (P=0.002) and
rose further after delivery (P=0.014), whereas leptin and insulin levels
decreased after parturition (both P<0.001). In conclusion, visfatin is
elevated in women with GDM and increases during the course of pregnancy as well
as after delivery. Furthermore, visfatin shows no association with insulin and
leptin in women with GDM.
Krzyzanowska et al. Clin Sci (Lond). 2006 May;110(5):605-9
Visfatin, a new adipokine, facilitates adipogenesis and has insulin-mimetic properties. We aimed to investigate the plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus (T2DM) and impaired glucose tolerance (IGT), who had no obesity or hypertension. Twenty-two patients with T2DM, 18 subjects with IGT and 40 healthy controls were enrolled. Visfatin levels were measured along with the BMI, blood pressure, lipids, glucose, insulin, adiponectin and hsCRP levels, and HOMA-IR indexes. Age, sex and BMI were similar in all groups. Visfatin levels were higher in the diabetic group than the controls (p=0.01). There was no significant difference in the visfatin levels between the T2DM and IGT groups as well as IGT group and healthy controls. Plasma visfatin concentrations did not differ between men and women. Visfatin levels did not correlate with BMI, blood pressure, plasma adiponectin, insulin, hsCRP, glucose and lipid levels or HOMA-IR indexes in the three groups. These results indicate that hyperglycemia causes an increase in plasma visfatin levels and, as in people with T2DM but not with IGT, this increase gets more prominent as the glucose intolerance worsens.
Dogru et al. Diabetes Res Clin Pract. 2007 Apr;76(1):24-9.
CONTEXT: Visfatin (also known as pre-B cell colony-enhancing factor or PBEF) is a cytokine that highly
expressed in visceral fat and whose blood levels correlate with obesity.
Originally isolated as a secreted factor that promote the growth of B cell
precursors and recently found to act as an insulin analogue on the insulin
receptor, its pathophysiologic role in humans remain largely
unknown.
OBJECTIVES: In this study we investigate whether or not plasma
visfatin level is altered in patients with type 2 diabetes mellitus (T2DM).
DESIGN AND PATIENTS: Plasma visfatin as well as adiponectin and resistin
concentrations were measured through ELISA in type 2 diabetic and non-diabetic
subjects.
RESULTS: A total of 61 patients with T2DM and 59 sex- and
age-matched non-diabetic subjects were studied. Plasma visfatin was found to be
elevated in patients with T2DM (31.9 ± 31.7 ng/mL vs. 15.8 ± 16.7 ng/mL, p=
0.002). In contrast, adiponectin was decreased (4.3 ± 2.5 μg/mL vs. 30.8 ± 10.3
μg/mL, p< 0.001), while plasma resistin level did not differ between the
groups. Increasing concentrations of visfatin were independently and
significantly associated with T2DM. Multiple logistic regression analysis
revealed visfatin as an independent association factor for T2DM even after full
adjustment of known biomarkers. The association between adiponectin and T2DM was
no longer significant after adjustments for BMI or WHR. In a multiple linear
regression analysis, only WHR was independently associated with plasma visfatin
level.
CONCLUSION: Our results indicate that visfatin may play a role in
the pathogenesis of T2DM.
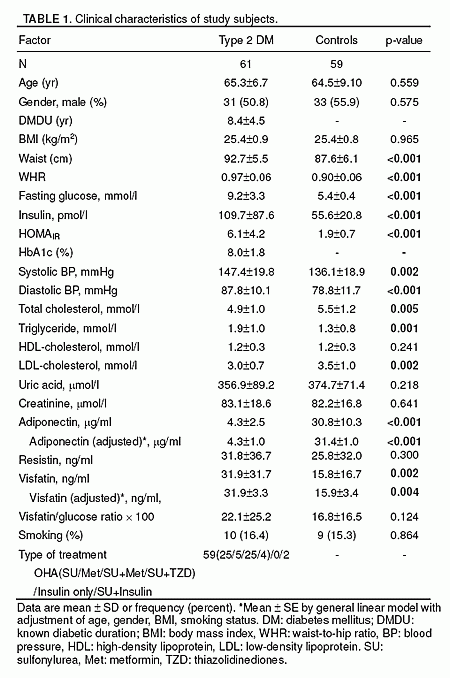
Chen et al. J Clin Endocrinol Metab. 2006 Jan;91(1):295-9.
CONTEXT: Visfatin was recently reported to be expressed in human adipose tissue and to exert insulin-mimicking
effects. Objective: To examine if visfatin is a true adipokine and expressed in
isolated fat cells. We also examined if visfatin is regulated by
thiazolidinediones and, thus, can contribute to the ability of these agents to
improve insulin sensitivity.
DESIGN: Open labeled drug therapy
trial.
SETTING: University Hospital.
PATIENTS: Seven newly diagnosed and previously untreated Type 2 diabetic patients and 6 healthy individuals with
reduced insulin sensitivity participated in the study.
INTERVENTION: Pioglitazone therapy, 30-45 mg per day for 3-4 weeks.
MAIN OUTCOME MEASURES: Serum and adipose tissue mRNA levels of visfatin and adiponectin.
RESULTS: Visfatin mRNA is expressed in both adipose tissue and isolated adipocytes.
Treatment with thiazolidinediones for 3-4 weeks did not alter the gene
expression or circulating levels of visfatin in either the non-diabetic or the
diabetic individuals, while adiponectin increased significantly.
CONCLUSION: The present study shows that visfatin is a true adipokine but it is not
regulated by TZD and, thus, is unlikely to contribute to the insulin-sensitizing
actions of these drugs.


Hammarstedt et al. J Clin Endocrinol Metab. 2006 Mar;91(3):1181-4.
CONTEXT AND OBJECTIVE: Visfatin is a peptide suggested to play a role in
glucose homeostasis. In the present study we investigated the role of genetic
variation in the visfatin gene in the pathophysiology of obesity/type 2 diabetes
mellitus (T2DM).
DESIGN: The visfatin gene (PBEF1) was
sequenced in DNA samples from 24 non-related Caucasian subjects. Identified
genetic variants were used for association analyses of T2DM in a case-control
study (503 diabetic subjects and 476 healthy controls) and T2DM-related traits
in 626 non-diabetic subjects. Effect of genetic variation in the visfatin gene
on its mRNA expression in a subgroup of 157 non-diabetic subjects with
measurements of visfatin mRNA expression in visceral and sc fat depots was also
analyzed.
RESULTS: Seven single nucleotide polymorphisms (SNPs) and one insertion/deletion were
identified. Three SNPs (rs9770242, -948G>T, rs4730153) which were
representatives of their linkage disequilibrium groups were genotyped in
Caucasians from Germany with a wide range of body fat distribution and insulin
sensitivity for association analyses. No association of T2DM with any of the
genotyped SNPs or their haplotypes was found. However, the ratio of visceral/sc
visfatin mRNA expression was associated with all 3 genetic polymorphisms
(P < 0.05). Moreover, the -948G>T variant was associated with 2 h plasma glucose and fasting insulin
concentrations (P < 0.05) in non-diabetic subjects.
CONCLUSIONS: In conclusion, our data suggest that genetic variation in the visfatin gene may
have a minor effect on visceral and sc visfatin mRNA expression profiles but
does not play a major role in the development of obesity or T2DM.

Böttcher et al. J Clin Endocrinol Metab. 2006 Jul;91(7):2725-31.
 
 
|
|
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
No primary antibody (pre-immuno serum) |
Pretreatment |
Intact |
Blocking |
2% Normal Goat Serum |
Primary Antibody |
Rabbit Anti-Visfatin (400-450) (Human) Antiserum (Catalog No.:H-003-84) |
Optimal Dilution |
1:200~500, 1 hour at RT |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification |
ABC (Vector) (1:400, 30 min) |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |
|
|
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
No primary antibody (pre-immuno serum) |
Pretreatment |
Intact |
Blocking |
2% Normal Goat Serum |
Primary Antibody |
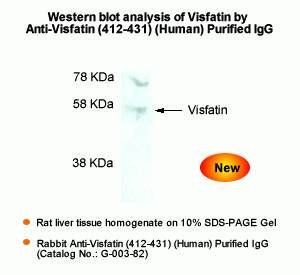
Rabbit Anti-Visfatin (412-431) (Human) Antiserum (Catalog No.:H-003-82) |
Optimal Dilution |
1:200~500, 1 hour at RT |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification |
ABC (Vector) (1:400, 30 min) |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |
|
|
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
No primary antibody (pre-immuno serum) |
Pretreatment |
Intact |
Blocking |
2% Normal Goat Serum |
Primary Antibody |
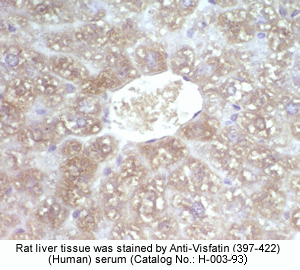
Rabbit Anti-Visfatin (397-422) (Human) Antiserum (Catalog No.:H-003-93) |
Optimal Dilution |
1:200, 1 hour at RT |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification |
ABC (Vector) (1:400, 30 min) |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |
 

Sample |
Dilution |
Observed (ng/ml) |
Expected (ng/ml) |
[Observed/Expected] x 100 (%) |
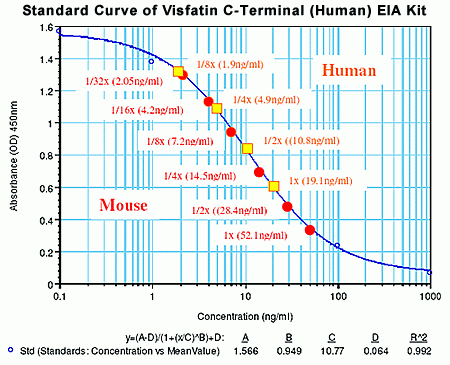
Non-extracted EDTA plasma from subjects (n=6) |
neat |
38.67 |
|
|
1/2 |
17.15 |
34.3 |
88.7 |
1/4 |
8.66 |
34.64 |
89.5 |
1/8 |
6.17 |
49.36 |
127.6 |
1/16 |
3.36 |
53.76 |
139.0 |
Non-extracted EDTA plasma from subjects (n=4) |
neat |
19.1 |
|
|
1/2 |
10.8 |
9.55 |
113.1 |
1/4 |
4.9 |
4.78 |
102.1 |
1/8 |
1.9 |
2.38 |
79.8 |
Non-extracted EDTA plasma from mouse (n=6) |
neat |
52.1 |
|
|
1/2 |
28.4 |
26.05 |
109.0 |
1/4 |
14.5 |
13.02 |
111.4 |
1/8 |
7.2 |
6.51 |
110.6 |
1/16 |
4.2 |
3.26 |
128.8 |
1/32 |
2.05 |
1.63 |
125.8 |

Wang et al. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide.
Cardiovasc Res. 2009 Feb 1;81(2):370-80.
Skokowa et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway.
Nat Med. 2009 Feb;15(2):151-8.
de Boer et al. Plasma levels of PBEF/Nampt/visfatin are decreased in patients with liver cirrhosis.
Am J Physiol Gastrointest Liver Physiol. 2009 Feb;296(2):G196-201.
du Luis et al. Relation of visfatin to cardiovascular risk factors and adipocytokines in patients with impaired fasting glucose.
Nutrition. 2009 Jan 6. [Epub ahead of print]
Li et al. The adipose triglyceride lipase, adiponectin and visfatin are downregulated by tumor necrosis factor-alpha (TNF-alpha) in vivo.
Cytokine. 2009 Jan;45(1):12-9.
Kato et al. Relationship between serum pre-B cell colony-enhancing factor/visfatin and atherosclerotic parameters in chronic hemodialysis patients.
Am J Nephrol. 2009;29(1):31-5.
Zhong et al. Increased serum visfatin in patients with metabolic syndrome and carotid atherosclerosis.
Clin Endocrinol (Oxf). 2008 Dec;69(6):878-84.
Retnakaran et al. Correlation of circulating full-length visfatin (PBEF/NAMPT) with metabolic parameters in subjects with and without diabetes: a cross-sectional study.
Clin Endocrinol (Oxf). 2008 Dec;69(6):885-93.
Younossi et al. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH).
Obes Surg. 2008 Nov;18(11):1430-7.
Storka et al. Angiotensin inhibition stimulates PPARgamma and the release of visfatin.
Eur J Clin Invest. 2008 Nov;38(11):820-6.
Panidis et al. Plasma visfatin levels in normal weight women with polycystic ovary syndrome.
Eur J Intern Med. 2008 Oct;19(6):406-12.
Dahmen et al. Elevated peripheral visfatin levels in narcoleptic patients.
PLoS ONE. 2008 Aug 20;3(8):e2980.
Botella-Carretero et al. The increase in serum visfatin after bariatric surgery in morbidly obese women is modulated by weight loss, waist circumference, and presence or absence of diabetes before surgery.
Obes Surg. 2008 Aug;18(8):1000-6.
Brema et al. Plasma visfatin is reduced after aerobic exercise in early onset type 2 diabetes mellitus.
Diabetes Obes Metab. 2008 Jul;10(7):600-2.
Palin et al. Visfatin expression is not associated with adipose tissue abundance in the porcine model.
Domest Anim Endocrinol. 2008 Jul;35(1):58-73.
Ibáñez et al. Gender specificity of body adiposity and circulating adiponectin, visfatin, insulin, and insulin growth factor-I at term birth: relation to prenatal growth.
J Clin Endocrinol Metab. 2008 Jul;93(7):2774-8.
Perez-Iglesias et al. Effect of antipsychotics on peptides involved in energy balance in drug-naive psychotic patients after 1 year of treatment.
J Clin Psychopharmacol. 2008 Jun;28(3):289-95.
de Luis et al. Effect of a hypocaloric diet on serum visfatin in obese non-diabetic patients.
Nutrition. 2008 Jun;24(6):517-21.
Ibáñez et al. Visceral adiposity without overweight in children born small for gestational age.
J Clin Endocrinol Metab. 2008 Jun;93(6):2079-83.
Yilmaz et al. Endothelial dysfunction in type-2 diabetics with early diabetic nephropathy is associated with low circulating adiponectin.
Nephrol Dial Transplant. 2008 May;23(5):1621-7.
Bouhours-Nouet et al. High birth weight and early postnatal weight gain protect obese children and adolescents from truncal adiposity and insulin resistance: metabolically healthy but obese subjects?
Diabetes Care. 2008 May;31(5):1031-6.
Krzysik-Walker et al. Is visfatin an adipokine or myokine? Evidence for greater visfatin expression in skeletal muscle than visceral fat in chickens.
Endocrinology. 2008 Apr;149(4):1543-50.
Matsui et al. Visfatin (pre-B cell colony-enhancing factor) gene expression in patients with rheumatoid arthritis.
Ann Rheum Dis. 2008 Apr;67(4):571-2.
Jarrar et al. Adipokines and cytokines in non-alcoholic fatty liver disease.
Aliment Pharmacol Ther. 2008 Mar 1;27(5):412-21.
Yilmaz et al. Serum visfatin concentration and endothelial dysfunction in chronic kidney disease.
Nephrol Dial Transplant. 2008 Mar;23(3):959-65.
Kendal-Wright et al. Chronic stretching of amniotic epithelial cells increases pre-B cell colony-enhancing factor (PBEF/visfatin) expression and protects them from apoptosis.
Placenta. 2008 Mar;29(3):255-65.
Rauchenzauner et al. Adiponectin and visfatin concentrations in children treated with valproic acid.
Epilepsia. 2008 Feb;49(2):353-7.
Choi et al. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease.
Eur J Endocrinol. 2008 Feb;158(2):203-7.
Peng et al. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men.
Clin Chim Acta. 2008 Jan;387(1-2):31-5.
Kostapanos et al. Effect of rosuvastatin treatment on plasma visfatin levels in patients with primary hyperlipidemia.
Eur J Pharmacol. 2008 Jan 14;578(2-3):249-52.
Filippatos et al. Increased plasma visfatin levels in subjects with the metabolic syndrome.
Eur J Clin Invest. 2008 Jan;38(1):71-2.
López-Bermejo et al. Cord serum visfatin at term birth: maternal smoking unmasks the relation to foetal growth.
Clin Endocrinol (Oxf). 2008 Jan;68(1):77-81.
Seo et al. Plasma visfatin levels are positively associated with circulating interleukin-6 in apparently healthy Korean women.
Diabetes Res Clin Pract. 2008 Jan;79(1):108-11.
Mazaki-Tovi et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition.
J Perinat Med. 2008;36(6):485-96.
Goldfine et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal.
J Clin Endocrinol Metab. 2007 Dec;92(12):4678-85.
Körner et al. Molecular characteristics of serum visfatin and differential detection by immunoassays.
J Clin Endocrinol Metab. 2007 Dec;92(12):4783-91.
Shea et al. Serum retinol-binding protein 4 concentrations in response to short-term overfeeding in normal-weight, overweight, and obese men.
Am J Clin Nutr. 2007 Nov;86(5):1310-5.
Oki et al. Circulating visfatin level is correlated with inflammation, but not with insulin resistance.
Clin Endocrinol (Oxf). 2007 Nov;67(5):796-800.
Garcia-Fuentes et al. Plasma Visfatin Concentrations in Severely Obese Subjects Are Increased After Intestinal Bypass
Obesity (Silver Spring). 2007 Oct;15(10):2391-5.
Choi et al. Effect of exercise training on plasma visfatin and eotaxin levels.
Eur J Endocrinol. 2007 Oct;157(4):437-42.
Wang et al. The circulating PBEF/NAMPT/visfatin level is associated with a beneficial blood lipid profile.
Pflugers Arch. 2007 Sep;454(6):971-6.
Chen et al. The relationship between visfatin levels and anthropometric and metabolic parameters: association with cholesterol levels in women
Metabolism. 2007 Sep;56(9):1216-20.
Brentano et al. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities.
Arthritis Rheum. 2007 Sep;56(9):2829-39.
Zahorska-Markiewicz et al. Serum concentration of visfatin in obese women.
Metabolism. 2007 Aug;56(8):1131-4.
Hsieh et al. Both slow-release and regular-form metformin improve glycemic control without altering plasma visfatin level in patients with type 2 diabetes mellitus.
Metabolism. 2007 Aug;56(8):1087-92.
Nüsken et al. Preanalytical influences on the measurement of visfatin by enzyme immuno assay.
Clin Chim Acta. 2007 Jul;382(1-2):154-6.
Haider et al. Visfatin response to glucose is reduced in women with gestational diabetes mellitus.
Diabetes Care. 2007 Jul;30(7):1889-91.
Malamitsi-Puchner et al. Perinatal circulating visfatin levels in intrauterine growth restriction.
Pediatrics. 2007 Jun;119(6):e1314-8.
Zhou et al. Tigogenin inhibits adipocytic differentiation and induces osteoblastic differentiation in mouse bone marrow stromal cells.
Mol Cell Endocrinol. 2007 May 30;270(1-2):17-22.
Ingelsson et al. Clinical correlates of circulating visfatin levels in a community-based sample.
Diabetes Care. 2007 May;30(5):1278-80.
Lewandowski et al. Elevated serum levels of visfatin in gestational diabetes: a comparative study across various degrees of glucose tolerance.
Diabetologia. 2007 May;50(5):1033-7.
Haider et al. Effect of rosiglitazone on visfatin and retinol-binding protein-4 plasma concentrations in HIV-positive patients.
Clin Pharmacol Ther. 2007 Apr;81(4):580-5.
Malamitsi-Puchner et al. Blood visfatin concentrations in normal full-term pregnancies.
Acta Paediatr. 2007 Apr;96(4):526-9.
Sandeep et al. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians.
Metabolism. 2007 Apr;56(4):565-70.
Takebayashi et al. Association between plasma visfatin and vascular endothelial function in patients with type 2 diabetes mellitus.
Metabolism. 2007 Apr;56(4):451-8.
Dogru et al. Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance.
Diabetes Res Clin Pract. 2007 Apr;76(1):24-9.
Fernández-Real et al. Circulating visfatin is associated with parameters of iron metabolism in subjects with altered glucose tolerance.
Diabetes Care. 2007 Mar;30(3):616-21.
Dogru et al. Plasma visfatin levels in young male patients with uncomplicated and newly diagnosed hypertension.
J Hum Hypertens. 2007 Feb;21(2):173-5.
Zhou et al. Macrostemonoside A promotes visfatin expression in 3T3-L1 cells.
Biol Pharm Bull. 2007 Feb;30(2):279-83.
Manco et al. Effect of massive weight loss on inflammatory adipocytokines and the innate immune system in morbidly obese women.
J Clin Endocrinol Metab. 2007 Feb;92(2):483-90.
Dahl et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization.
Circulation. 2007 Feb 27;115(8):972-80.
Sun et al. Serum visfatin concentrations are positively correlated with serum triacylglycerols and down-regulated by overfeeding in healthy young men.
Am J Clin Nutr. 2007 Feb;85(2):399-404.
Varma et al. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipids, and inflammation.
J Clin Endocrinol Metab. 2007 Feb;92(2):666-72.
Moschen et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties.
J Immunol. 2007 Feb 1;178(3):1748-58.
Frydelund-Larsen et al. Visfatin mRNA expression in human subcutaneous adipose tissue is regulated by exercise.
Am J Physiol Endocrinol Metab. 2007 Jan;292(1):E24-31.
Yonezawa et al. Visfatin is present in bovine mammary epithelial cells, lactating mammary gland and milk, and its expression is regulated by cAMP pathway.
FEBS Lett. 2006 Dec 11;580(28-29):6635-43.
Dimitriadis et al. Insulin action in adipose tissue and muscle in hypothyroidism.
J Clin Endocrinol Metab. 2006 Dec;91(12):4930-7.
Tan et al. Increased visfatin messenger ribonucleic acid and protein levels in adipose tissue and adipocytes in women with polycystic ovary syndrome: parallel increase in plasma visfatin.
J Clin Endocrinol Metab. 2006 Dec;91(12):5022-8.
Krzyzanowska et al. Increase in visfatin after weight loss induced by gastroplastic surgery.
Obesity (Silver Spring). 2006 Nov;14(11):1886-9.
Haider et al. Exercise training lowers plasma visfatin concentrations in patients with type 1 diabetes.
J Clin Endocrinol Metab. 2006 Nov;91(11):4702-4.
Haider et al. Free fatty acids normalize a rosiglitazone-induced visfatin release.
Am J Physiol Endocrinol Metab. 2006 Nov;291(5):E885-90.
López-Bermejo et al. Serum visfatin increases with progressive beta-cell deterioration.
Diabetes. 2006 Oct;55(10):2871-5.
Haider et al. The adipokine visfatin is markedly elevated in obese children.
J Pediatr Gastroenterol Nutr. 2006 Oct;43(4):548-9.
Otero et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis.
Ann Rheum Dis. 2006 Sep;65(9):1198-201.
Jian et al. The visfatin gene is associated with glucose and lipid metabolism in a Chinese population.
Diabet Med. 2006 Sep;23(9):967-73.
Schindler et al. Impact of antiretroviral therapy on visfatin and retinol-binding protein 4 in HIV-infected subjects.
Eur J Clin Invest. 2006 Sep;36(9):640-6.
Haider et al. The release of the adipocytokine visfatin is regulated by glucose and insulin.
Diabetologia. 2006 Aug;49(8):1909-14.
Pagano et al. Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humans.
J Clin Endocrinol Metab. 2006 Aug;91(8):3165-70.
Nowell et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis.
Arthritis Rheum. 2006 Jul;54(7):2084-95.
Böttcher et al. Genetic variation in the visfatin gene (PBEF1) and its relation to glucose metabolism and fat-depot-specific messenger ribonucleic acid expression in humans.
J Clin Endocrinol Metab. 2006 Jul;91(7):2725-31.
Krzyzanowska et al. Increased visfatin concentrations in women with gestational diabetes mellitus.
Clin Sci (Lond). 2006 May;110(5):605-9.
Haider et al. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding.
J Clin Endocrinol Metab. 2006 Apr;91(4):1578-81.
Curat et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin.
Diabetologia. 2006 Apr;49(4):744-7.
Hammarstedt et al. Visfatin is an adipokine, but it is not regulated by thiazolidinediones.
J Clin Endocrinol Metab. 2006 Mar;91(3):1181-4.
Chen et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus.
J Clin Endocrinol Metab. 2006 Jan;91(1):295-9.
Berndt et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans.
Diabetes. 2005 Oct;54(10):2911-6.
Chan et al. Adiponectin and other adipocytokines as predictors of markers of triglyceride-rich lipoprotein metabolism.
Clin Chem. 2005 Mar;51(3):578-85.
|
|
|
%visfatin%
|
|
|


