The Notch receptor and its ligands are key components in a core metazoan signaling pathway that regulates the spatial patterning, timing and outcome of many cell-fate decisions. Ligands contain a disulfide-rich Delta/Serrate/LAG-2 (DSL) domain required for Notch trans-activation or cis-inhibition. Here we report the X-ray structure of a receptor binding region of a Notch ligand, the DSL-EGF3 domains of human Jagged-1 (J-1(DSL-EGF3)). The structure reveals a highly conserved face of the DSL domain, and we show, by functional analysis of Drosophila melanogster ligand mutants, that this surface is required for both cis- and trans-regulatory interactions with Notch. We also identify, using NMR, a surface of Notch-1 involved in J-1(DSL-EGF3) binding. Our data imply that cis- and trans-regulation may occur through the formation of structurally distinct complexes that, unexpectedly, involve the same surfaces on both ligand and receptor.
Cordle J, Johnson S, Tay JZ, et al., Nat Struct Mol Biol. 2008 Aug;15(8):849-57. doi: 10.1038/nsmb.1457. Epub 2008 Jul 27.
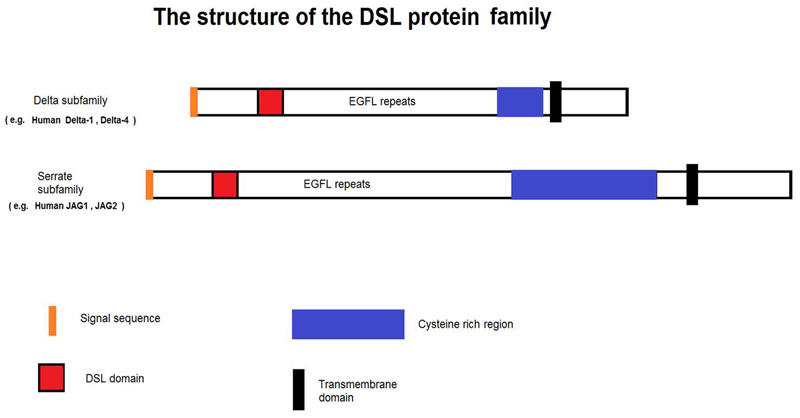
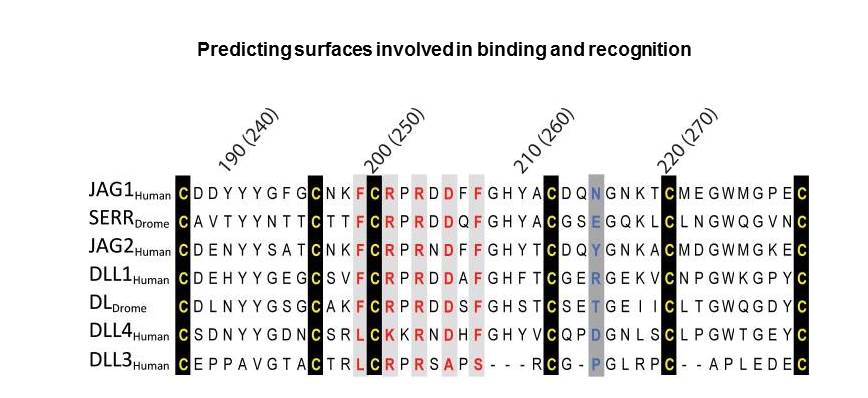
Analysis of an alignment of Jagged/Delta family DSL domains representing a variety of species
H. sapiens Jagged-1, residues 187-229;
D. melanogaster Serrate, residues 237-279;
H. sapiens Jagged-2, residues 198-240;
H. sapiens Delta-like 1, residues 179-221;
D. melanogaster Delta, residues 184-226;
H. sapiens Delta-like 4, residues 175-217;
H. sapiens Delta-like 3, residues 178-215


