|
|
MRAP2 |
the protein associate with obesity
|
Accessory to Obesity?
Science, AAAS, Washington, DC 20005, USA
Melanocortin receptors are a family of cell membrane receptors that control diverse physiological functions. Mutations in the gene encoding melanocortin 4 receptor (MC4R) are a cause of familial early-onset obesity. Asai et al. studied the function of an accessory protein for MC4R signaling, MRAP2, and found that mice genetically deficient in MRAP2 develop severe obesity. Sequencing of MRAP2 in unrelated, severely obese humans revealed one individual with a clearly disruptive genetic variant, suggesting that MRAP2 mutations might also be a rare cause of human obesity. In a zebrafish model, Sebag et al. studied two paralogs of the MRAP2 accessory protein, one of which enhanced MC4R responsiveness to α–melanocyte-stimulating hormone, which regulates feeding and growth.
Related articles ():
1.M. Asai, S. Ramachandrappa, M. Joachim, et al., Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 341, 275–278 (2013).
2.J. A. Sebag, C. Zhang, P. M. Hinkle, et al., Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science 341, 278–281 (2013).
3.Novoselova TV, Jackson D, Campbell DC, et al., Melanocortin receptor accessory proteins in adrenal gland physiology and beyond. J Endocrinol. 2013 Mar 19;217(1):R1-11. doi: 10.1530/JOE-12-0501. Print 2013 Apr.
4. Julien A. Sebag and Patricia M. Hinkle (6 April 2010), Regulation of G Protein–Coupled Receptor Signaling: Specific Dominant-Negative Effects of Melanocortin 2 Receptor Accessory Protein 2 (MRAP2). Sci. Signal. 3 (116), ra28. [DOI: 10.1126/scisignal.2000593] [Abstract]
Melanocortin receptor accessory proteins (MRAPs) modulate signaling of melanocortin receptors in vitro. To investigate the physiological role of brain-expressed melanocortin 2 receptor accessory protein 2 (MRAP2), we characterized mice with whole-body and brain-specific targeted deletion of Mrap2, both of which develop severe obesity at a young age. Mrap2 interacts directly with melanocortin 4 receptor (Mc4r), a protein previously implicated in mammalian obesity, and it enhances Mc4r-mediated generation of the second messenger cyclic adenosine monophosphate, suggesting that alterations in Mc4r signaling may be one mechanism underlying the association between Mrap2 disruption and obesity. In a study of humans with severe, early-onset obesity, we found four rare, potentially pathogenic genetic variants in MRAP2, suggesting that the gene may also contribute to body weight regulation in humans.
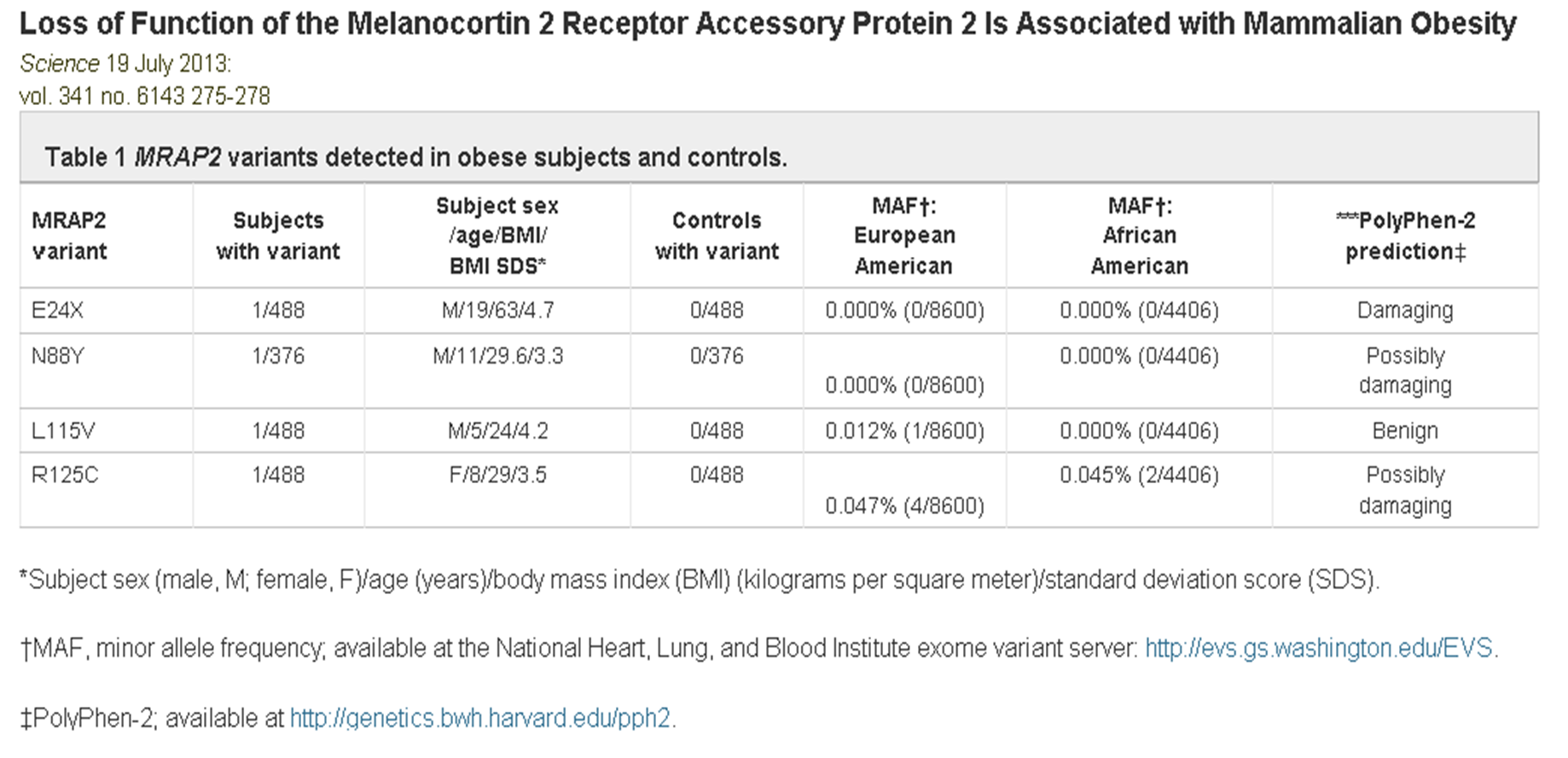
Asai M, Ramachandrappa S, Joachim M et al., Science. 2013 Jul 19;341(6143):275-8. doi: 10.1126/science.1233000.
The melanocortin-4 receptor (MC4R) is essential for control of energy homeostasis in vertebrates. MC4R interacts with melanocortin receptor accessory protein 2 (MRAP2) in vitro, but its functions in vivo are unknown. We found that MRAP2a, a larval form, stimulates growth of zebrafish by specifically blocking the action of MC4R. In cell culture, this protein binds MC4R and reduces the ability of the receptor to bind its ligand, α-melanocyte-stimulating hormone (α-MSH). A paralog, MRAP2b, expressed later in development, also binds MC4R but increases ligand sensitivity. Thus, MRAP2 proteins allow for developmental control of MC4R activity, with MRAP2a blocking its function and stimulating growth during larval development, whereas MRAP2b enhances responsiveness to α-MSH once the zebrafish begins feeding, thus increasing the capacity for regulated feeding and growth.
Sebag JA, Zhang C, Hinkle PM et al., Science. 2013 Jul 19;341(6143):278-81. doi: 10.1126/science.1232995.
The melanocortin receptor (MCR) family consists of five G-protein-coupled receptors (MC1R-MC5R) with diverse physiological roles. MC1R controls pigmentation, MC2R is a critical component of the hypothalamic-pituitary-adrenal axis, MC3R and MC4R have a vital role in energy homeostasis and MC5R is involved in exocrine function. The melanocortin receptor accessory protein (MRAP) and its paralogue MRAP2 are small single-pass transmembrane proteins that have been shown to regulate MCR expression and function. In the adrenal gland, MRAP is an essential accessory factor for the functional expression of the MC2R/ACTH receptor. The importance of MRAP in adrenal gland physiology is demonstrated by the clinical condition familial glucocorticoid deficiency, where inactivating MRAP mutations account for ∼20% of cases. MRAP is highly expressed in both the zona fasciculata and the undifferentiated zone. Expression in the undifferentiated zone suggests that MRAP could also be important in adrenal cell differentiation and/or maintenance. In contrast, the role of adrenal MRAP2, which is highly expressed in the foetal gland, is unclear. The expression of MRAPs outside the adrenal gland is suggestive of a wider physiological purpose, beyond MC2R-mediated adrenal steroidogenesis. In vitro, MRAPs have been shown to reduce surface expression and signalling of all the other MCRs (MC1,3,4,5R). MRAP2 is predominantly expressed in the hypothalamus, a site that also expresses a high level of MC3R and MC4R. This raises the intriguing possibility of a CNS role for the MRAPs.
Novoselova TV, Jackson D, Campbell DC et al., J Endocrinol. 2013 Mar 19;217(1):R1-11. doi: 10.1530/JOE-12-0501. Print 2013 Apr.
Heterotrimeric guanine nucleotide-binding protein (G protein)-coupled receptors (GPCRs), which constitute the largest family of membrane proteins, mediate responses to diverse physiological stimuli. The presence of melanocortin 2 receptors (MC2Rs) on the plasma membrane requires the presence of either MC2R accessory protein (MRAP) or MRAP2, which are homologous accessory proteins. Here, we show that, whereas MRAP was essential for activation of MC2R signaling, MRAP2 was an endogenous inhibitor that competed with MRAP for binding to MC2R and decreased the potency of adrenocorticotropic hormone (ACTH), the endogenous agonist for MC2Rs, in stimulating the production of adenosine 3',5'-monophosphate (cAMP). ACTH bound with high affinity to MC2Rs in the presence of MRAP, but not MRAP2. The ability of MRAP and MRAP2 to influence ligand-binding affinity was specific to MC2R, because these proteins had little effect on the binding of NDP-alpha-melanocyte-stimulating hormone to MC4R or on its stimulation of cAMP responses. These results demonstrate that the balance of stimulatory and inhibitory accessory proteins can control the sensitivity of a GPCR to its natural agonist.
Sebag JA, Hinkle PM., Sci Signal. 2010 Apr 6;3(116):ra28. doi: 10.1126/scisignal.2000593.
The melanocortin receptor (MCR) family consists of 5 G protein-coupled receptors (MC1R-MC5R) with diverse physiologic roles. MC2R is a critical component of the hypothalamic-pituitary-adrenal axis, whereas MC3R and MC4R have an essential role in energy homeostasis. Mutations in MC4R are the single most common cause of monogenic obesity. Investigating the way in which these receptors signal and traffic to the cell membrane is vital in understanding disease processes related to MCR dysfunction. MRAP is an MC2R accessory protein, responsible for adrenal MC2R trafficking and function. Here we identify MRAP2 as a unique homologue of MRAP, expressed in brain and the adrenal gland. We report that MRAP and MRAP2 can interact with all 5 MCRs. This interaction results in MC2R surface expression and signaling. In contrast, MRAP and MRAP2 can reduce MC1R, MC3R, MC4R, and MC5R responsiveness to [Nle4,D-Phe7]alpha-melanocyte-stimulating hormone (NDP-MSH). Collectively, our data identify MRAP and MRAP2 as unique bidirectional regulators of the MCR family.
Chan LF, Webb TR, Chung TT et al., Proc Natl Acad Sci U S A. 2009 Apr 14;106(15):6146-51. doi: 10.1073/pnas.0809918106. Epub 2009 Mar 27.
The melanocortin-2-receptor (MC(2) receptor), also known as the ACTH receptor, is a critical component of the hypothalamic-pituitary-adrenal axis. The importance of MC(2) receptor in adrenal physiology is exemplified by the condition familial glucocorticoid deficiency, a potentially fatal disease characterised by isolated cortisol deficiency. MC(2)receptor mutations cause ~25% of cases. The discovery of a MC(2) receptor accessory protein MRAP, mutations of which account for ~15%-20% of familial glucocorticoid deficiency, has provided insight into MC(2) receptor trafficking and signalling. MRAP is essential for the functional expression of MC(2) receptor. MRAP2, a novel homolog of MRAP, can also facilitate MC(2) receptor cell surface expression and function. Like MRAP, MRAP2 is a small transmembrane domain glycoprotein capable of homodimerising. In addition, MRAP/MRAP2 can heterodimerise. The presence of MRAP2 adrenal expression suggests a possible role for MRAP2 in adrenal physiology, which has yet to be elucidated. Importantly, new data shows that the MRAPs can interact with all the other melanocortin receptors (MC(1,3,4,5) receptor). In contrast to MC(2) receptor, this interaction results in reduced melanocortin receptor surface expression and signalling. MRAP2 is predominantly expressed in brain. Hypothalamic expression has been demonstrated for both MRAP and MRAP2. The ability of MRAPs to modulate different members of the melanocortin receptor family in a bidirectional manner is intriguing. Furthermore, central nervous system expression of MRAPs points to a role beyond MC(2) receptor mediated adrenal steroidogenesis.…
Chan LF, Metherell LA, Clark AJ., Eur J Pharmacol. 2011 Jun 11;660(1):171-80. doi: 10.1016/j.ejphar.2010.11.041. Epub 2011 Jan 3.
|
|
|
%MRAP2%
|
|
|


