Gene structure of CCK and CCKsv. Human CCK gene has two exons and one intron. Wild type CCK protein is encoded by exon1 and exon2 with exon1 containing the signal peptide (sp) and exon2 containing the mature peptide region (stripped bar). CCKsv is encoded by exon1, followed by a read through into the intron region, leading to the generation of novel mature region (hatched bar), followed by a stop codon and a new poly-adenylation signal.
Deng C, and Hsueh AJ. PLoS One. 2013 May 28;8(5):e64610. doi: 10.1371/journal.pone.0064610. Print 2013.
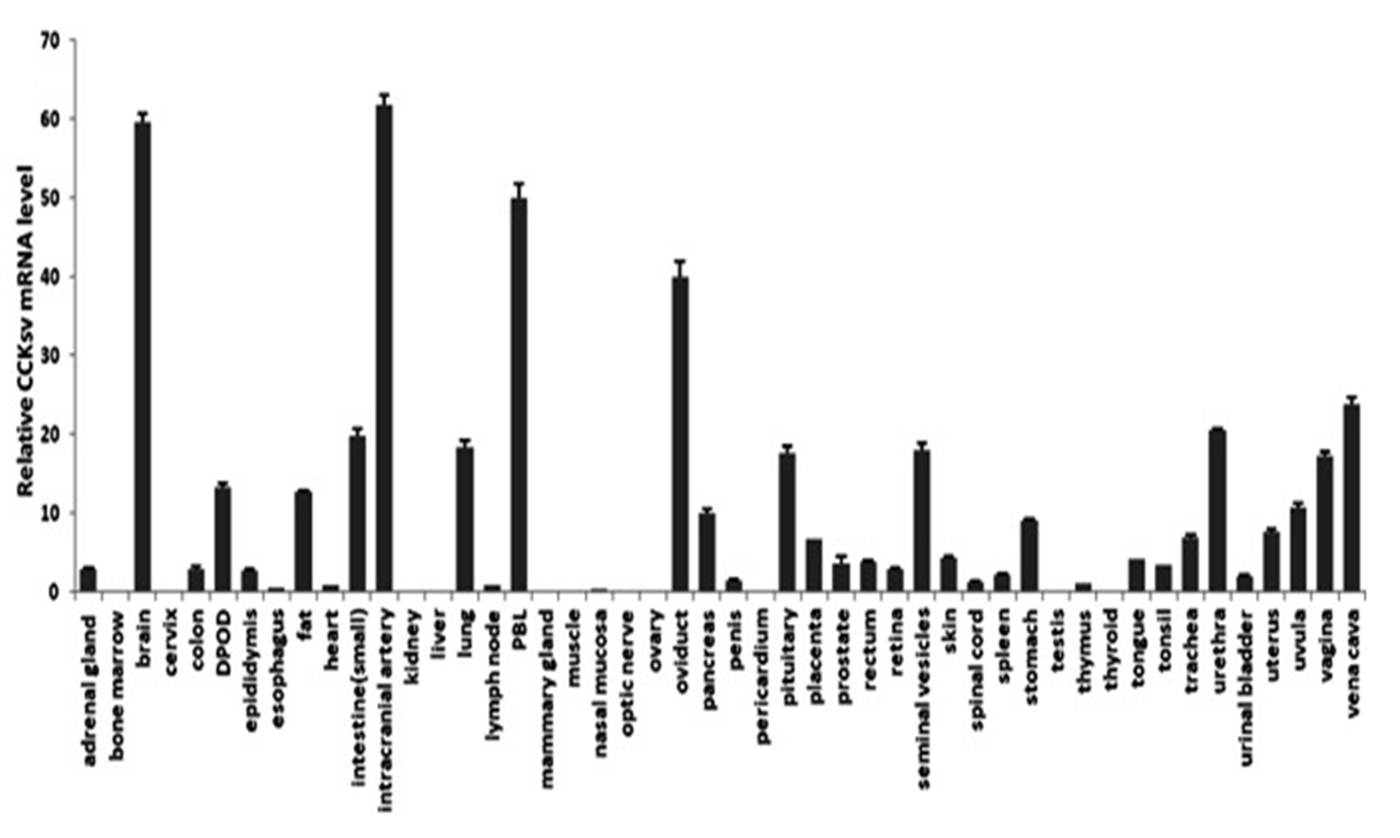
Alternative splicing of genes generates novel mRNAs, leading to the evolution of new functional proteins. Cholecystokinin (CCK) induces the release of pancreatic enzymes and the contraction of the gallbladder to promote the digestion of fat and proteins. CCK activates two G-protein-coupled receptors, CCKA and CCKB. Here, we showed that a CCKsv (splicing variant), originated de novo during Catarrhini evolution by including a portion of intronic sequence of the CCK gene, encodes novel C-terminal peptide sequence followed by a new poly-adenylation signal. CCKsv is expressed in many human tissues and likely a secreted peptide retaining the original signal peptide and the N-terminal proteolytic processing signal, together with novel C-terminal sequences. Although CCKsv cannot activate CCK receptors, it partially inhibits the CRE- or SRF-driven reporter activities stimulated by wide type CCK-8 mediated by both CCK receptors. Co-treatment with CCKsv also partially antagonizes Ewing tumor cell growth stimulated by CCK-8. Our study provides an example of new peptide hormone antagonist evolution in primates.
Deng C, and Hsueh AJ. PLoS One. 2013 May 28;8(5):e64610. doi: 10.1371/journal.pone.0064610. Print 2013.

Encoded protein sequence of CCKsv showed sequence homology with the C-terminal end of wild type CCK and the V5 taged-peptide (molecular weight < 5 kD) was found to be secreted and processed in CCKsv over-expressed SK-N-MC cells.


