|
|
|
Adropin | A Peptide Controlling Glucose and Lipid Homeostasis |
Aging-induced arterial stiffening is reduced by aerobic exercise training, and elevated production of nitric oxide (NO) participates in this effect. Adropin is a regulator of endothelial NO synthase and NO release, and circulating adropin level decreases with age. However, the effect of habitual aerobic exercise on circulating adropin levels in healthy middle-aged and older adults remains unclear. We sought to determine whether serumadropin level is associated with exercise training-induced changes in arterial stiffness. First, in a cross-sectional study, we investigated the association between serum adropin level and both arterial stiffness and cardiorespiratory fitness in 80 healthy middle-aged and older subjects (65.6 ± 0.9 yr). Second, in an intervention study, we examined the effects of 8-wk aerobic exercise training on serum adropin level and arterial stiffness in 40 healthy middle-aged and older subjects (67.3 ± 1.0 yr) divided into two groups: aerobic exercise training and sedentary controls. In the cross-sectional study, serum adropin level was negatively correlated with carotid β-stiffness (r = -0.437, P < 0.001) and positively correlated with plasma NOx level (r = 0.493, P < 0.001) and cardiorespiratory fitness (r = 0.457, P < 0.001). Serum adropin levels were elevated after the 8-wk aerobic exercise training intervention, and training-induced changes in serum adropin level were correlated with training-induced changes in carotid β-stiffness (r = -0.399, P < 0.05) and plasma NOx level (r = 0.623, P < 0.001). Thus the increase in adropin may participate in the exercise-induced reduction of arterial stiffness.
Fujie S, Hasegawa N, Sato K et al., Am J Physiol Heart Circ Physiol. 2015 Nov 15;309(10):H1642-7. doi: 10.1152/ajpheart.00338.2015. Epub 2015 Sep 14.
Adropin is a recently identified bioactive protein that promotes energy homeostasis by affecting glucose and lipid metabolism. Recently, adropin has also been reported to be associated with endothelial dysfunction. Also, ET-1, as a biomarker for endothelial dysfunction, is a key regulator in hypertension. Accordingly, the aim of the present study was to detect the relationship between plasma adropin and ET-1 levels in hypertension.A total of 123 participants, diagnosed with primary hypertension on the basis of World Health Organization criteria (systolic blood pressure [SBP] ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg), and 58 normotensive subjects were enrolled in the cross-sectional study from October 2011 to December 2013. All study participants were older than 18 years of age. Adropin and ET-1 levels were measured by enzyme-linked immunosorbent assay (ELISA). We found that plasma adropin levels were significantly lower in hypertensives compared with controls (3.18 ± 1.00 vs 4.21±1.14 ng/mL, P<0.001). Plasma ET-1 levels were higher in hypertensives than controls (2.60±1.14 vs 1.54±0.66pg/mL, P<0.001). Adropin had a negative correlation with DBP (r=-0.40, P<0.001), SBP (r=-0.49, P<0.001), and adjusted for age, body mass index, SBP, DBP, glucose, TC, TG, LDL, and Cr, there was a negative correlation between ET-1 and adropin (r=-0.20, P=0.04). In multivariate logistic regression analysis of the variables, ET-1 (odds ratio [OR], 3.84; 95% CI, 2.16-6.81; P<0.001) and adropin (OR, 0.99; 95% CI, 0.99 -1.0; P<0.001) were found to be independent predictors for hypertension.In conclusion, decreased plasma adropin levels are associated with increased blood pressure in hypertension. Adropin is an independent predictor for hypertension, and may influence blood pressure by protecting endothelial function.
Gu X, Li H, Zhu X et al., Medicine 2015 Oct;94(40):e1712. doi: 10.1097/MD.0000000000001712.
PURPOSE: Saphenous vein graft disease (SVGD), defined as an occlusion of 50% or more of the SVG excluding distal anastomotic occlusion, is an important predictor of morbidity after coronary artery bypass grafting (CABG). Late graft occlusion is a serious complication that often limits the use of the saphenous vein as a coronary bypass graft. Late graft occlusion is particularly common in old, degenerated venous grafts with advanced atherosclerotic plaques. Adropin has been implicated in the homeostatic control of metabolism. The purpose of this study was to investigate whether serum adropin levels are associated with late SVGD following CABG.
METHODS: Thirty-eight patients with SVGD involving at least one graft (occluded group; 14 females, 24 males) and 42 patients with a patent saphenous vein graft (patent group; 15 females, 27 males) were enrolled in this study. Venous blood samples were taken from all of the participants to measure plasma adropin levels using an enzyme-linked immunsorbent assay kit.
RESULTS: The mean adropin level was significantly lower in the occluded group than in the patent group (3.2 ± 0.71 vs. 4.9 ± 1.51 ng/mL, p < 0.001). Multivariate regression analysis showed that the adropin level was the independent predictor of late saphenous vein graft occlusion.
CONCLUSIONS: Adropin levels are lower in patients with late saphenous vein graft occlusion and these reduced adropin levels, together with other factors, may lead to saphenous vein graft occlusion. Larger and prospective studies are needed to determine if adropin plays a role in the pathogenesis of SVGD.
Demircelik B, Cakmak M, Nazli Y, et al, Clin Invest Med. 2014 Oct 4;37(5):E338-44.

|
Obesity and nutrient homeostasis are linked by mechanisms that are not fully elucidated. Here we describe a secreted protein, adropin, encoded by a gene, Energy Homeostasis Associated (Enho), expressed in liver and brain. Liver Enho expression is regulated by nutrition: lean C57BL/6J mice fed high-fat diet (HFD) exhibited a rapid increase, while fasting reduced expression compared to controls. However, liver Enho expression declines with diet-induced obesity (DIO) associated with 3 months of HFD or with genetically induced obesity, suggesting an association with metabolic disorders in the obese state. In DIO mice, transgenic overexpression or systemic adropin treatment attenuated hepatosteatosis and insulin resistance independently of effects on adiposity or food intake. Adropin regulated expression of hepatic lipogenic genes and adipose tissue peroxisome proliferator-activated receptor gamma, a major regulator of lipogenesis. Adropin may therefore be a factor governing glucose and lipid homeostasis, which protects against hepatosteatosis and hyperinsulinemia associated with obesity.
Kumar KG, et al. Cell Metab. 2008 Dec;8(6):468-81.
|

|
Obesity and nutrient homeostasis are linked by mechanisms that are not fully elucidated. Here we describe a secreted protein, adropin, encoded by a gene, Energy Homeostasis Associated (Enho), expressed in liver and brain. Liver Enho expression is regulated by nutrition: lean C57BL/6J mice fed high-fat diet (HFD) exhibited a rapid increase, while fasting reduced expression compared to controls. However, liver Enho expression declines with diet-induced obesity (DIO) associated with 3 months of HFD or with genetically induced obesity, suggesting an association with metabolic disorders in the obese state. In DIO mice, transgenic overexpression or systemic adropin treatment attenuated hepatosteatosis and insulin resistance independently of effects on adiposity or food intake. Adropin regulated expression of hepatic lipogenic genes and adipose tissue peroxisome proliferator-activated receptor gamma, a major regulator of lipogenesis. Adropin may therefore be a factor governing glucose and lipid homeostasis, which protects against hepatosteatosis and hyperinsulinemia associated with obesity.
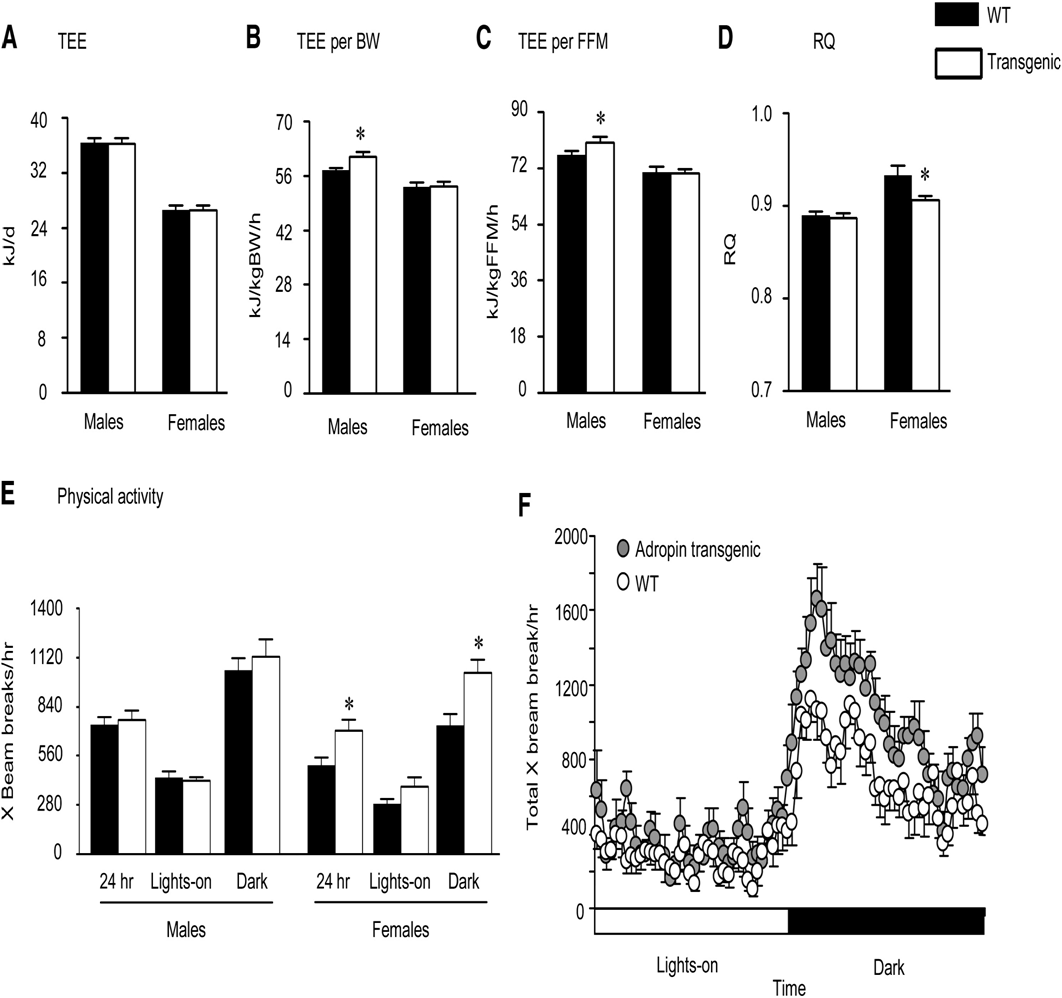
Analysis of Whole Body Energy Metabolism in Chow-Fed Adr-Tg and WT Mice
(A) Total 24 hr energy expenditure (TEE) of male and female Adr-Tg mice compared to controls.
(B and C) TEE expressed per g body weight (B) or FFM (C) (*p < 0.05).
(D) RQ of male and female Adr-Tg and WT mice (*p < 0.05).
(E and F) Spontaneous locomotory activity of female Adr-Tg and WT mice (*p < 0.05). In (E), the data are presented as mean X beam breaks over 24 hr, during the lights-on period (0600-1800 hr; L or white bar) and during the dark period (1800-0600 hr; D or black bar), by gender. All data are mean SEM and are the mean from 3 days of recordings; n = 7 each genotype and sex.
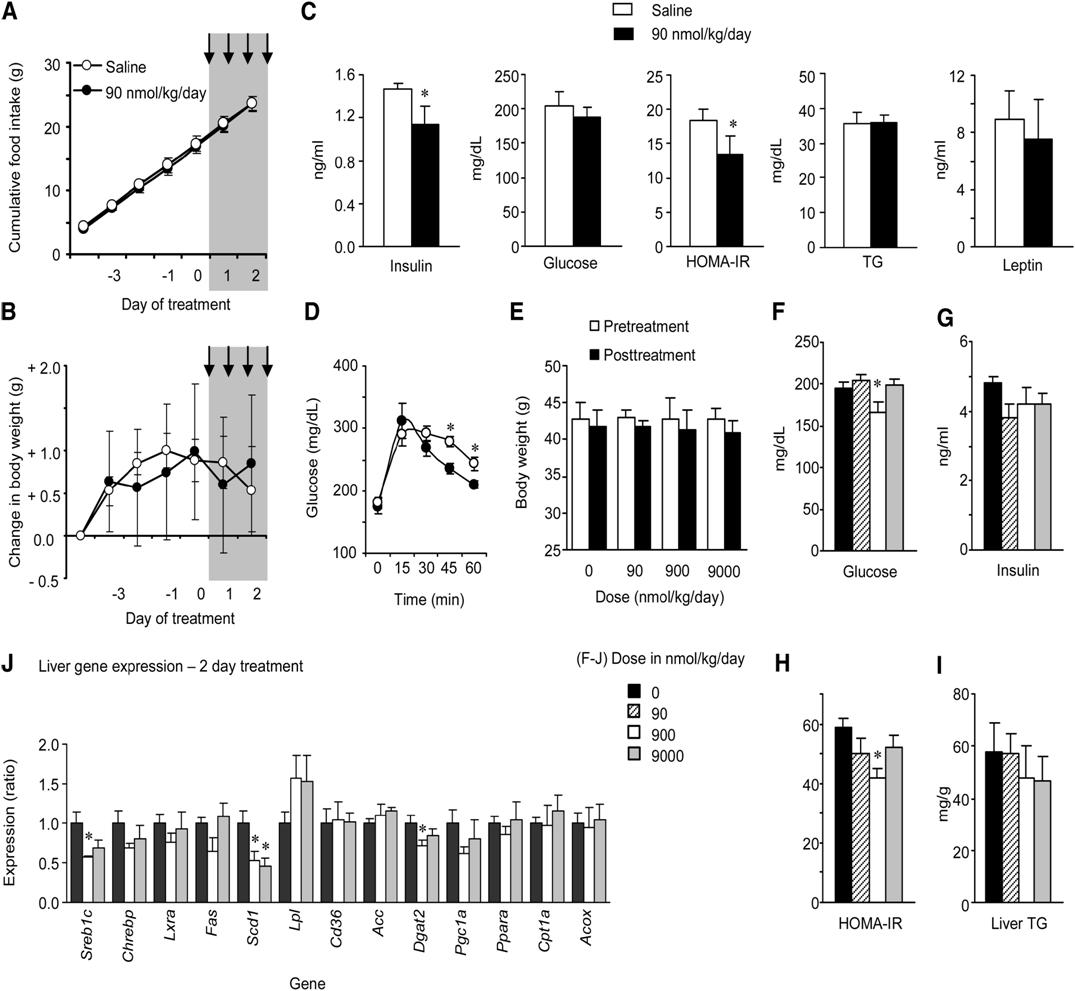
Short-Term Treatment with Synthetic Adropin(34-76) Improves Glucose Homeostasis in DIO B6 Mice Independently of Weight Loss or Reduced Food Intake
(A-D) Treatment of male DIO mice with 90 nmol/kg/day adropin(34-76) administered as two i.p. injections/day over 2 days did not significantly affect food intake (A) or weight gain (B) (n = 8/group). Treatment followed a 5 day lead-in period, with the injection period marked by the gray area. Following treatment, there were significant improvements in glucose homeostasis, indicated by reductions in fasting insulin and HOMA-IR values (C) and improved glucose tolerance (D). *p < 0.05 compared to saline. However, there were no changes observed in serum TG (C).
(E-J) A second study assessed a dose-response to 2 days of injections on glucose homeostasis (F-H) and liver metabolism (I, J) (n = 6/group). There was no significant effect of treatment on body weight (E, the white and solid bars are body weight pre- and posttreatment). There was evidence for improved glucose homeostasis (F-H), with significant reductions in fasting glucose (F) and HOMA-IR (H). While liver TG content was not significantly affected by treatment (I), there were changes in the expression of genes involved in fatty acid metabolism, which was statistically significant for Scd1 and diacylglycerol O-transferase 2 (Dgat2) (J). * p < 0.05 versus saline. The values shown are mean ± SEM.

Adropin(34-76) Therapy Affects Energy Metabolism in DIO B6 Mice and Cultured Adipocytes
(A-J) Administration of adropin(34-76) by i.p. injection at 0900 and 1800 hr for 14 days is associated with reduced food intake (A and B) and weight loss (C). Adropin treatment reduced fasting insulin (D) without affecting blood glucose (E), and increased serum adiponectin (F), suggesting improved insulin sensitivity. Adropin treatment improves hepatic steatosis (G) and significantly reduces liver TG accumulation (H), associated with reduced expression of genes involved in lipid synthesis in liver (I). Pparg expression in WAT of mice treated with adropin(34-76) was not significantly different from controls treated with saline (J). The values shown are mean ± SEM with N = 12/treatment group.
Kumar KG, et al. Cell Metab. 2008 Dec;8(6):468-81.
|

|
Adropin is a recently identified protein that has been implicated in the maintenance of energy homeostasis and insulin resistance. Because vascular function and insulin sensitivity are closely related, we hypothesized that adropin may also exert direct effects on the endothelium. In vitro cell culture models were partnered with an in vivo murine injury model to determine the potential vascular effects of adropin. Adropin was expressed in human umbilical vein and coronary artery endothelial cells (ECs). Adropin-treated endothelial cells exhibited greater proliferation, migration and capillary-like tube formation and less permeability and tumor necrosis factor-a-induced apoptosis. In keeping with a vascular protective effect, adropin stimulated Akt Ser(473) and endothelial nitric oxide (NO) synthase Ser(1177) phosphorylation. The former was abrogated in the presence of the phosphatidylinositol 3-kinase inhibitor LY294002, whereas the latter was attenuated by LY294002 and by mitogen-activated protein kinase kinase 1 inhibition with PD98059. Together, these findings suggest that adropin regulates NO bioavailability and events via the phosphatidylinositol 3-kinase-Akt and extracellular signal regulated kinase 1/2 signaling pathways. Adropin markedly upregulated vascular endothelial growth factor receptor-2 (VEGFR2) transcript and protein levels, and in VEGFR2-silenced endothelial cells, adropin failed to induce phosphorylation of endothelial NO synthase, Akt, and extracellular signal regulated kinase 1/2, supporting VEGFR2 as an upstream target of adropin-mediated endothelial NO synthase activation. Last, adropin improved murine limb perfusion and elevated capillary density following induction of hindlimb ischemia.
We report a potential endothelial protective role of adropin that is likely mediated via upregulation of endothelial NO synthase expression through the VEGFR2-phosphatidylinositol 3-kinase-Akt and VEGFR2-extracellular signal regulated kinase 1/2 pathways. Adropin represents a novel target to limit diseases characterized by endothelial dysfunction in addition to its favorable metabolic profile.
Lovren F. et al. Circulation 2010 Sep 14;122(11 Suppl):S185-92
(Note: this publication uses the bioactive peptide, Adropin, from Phoenix)
|

|
Adropin is a recently identified protein that has been implicated in the maintenance of energy homeostasis and insulin resistance. Because vascular function and insulin sensitivity are closely related, we hypothesized that adropin may also exert direct effects on the endothelium. In vitro cell culture models were partnered with an in vivo murine injury model to determine the potential vascular effects of adropin. Adropin was expressed in human umbilical vein and coronary artery endothelial cells (ECs). Adropin-treated endothelial cells exhibited greater proliferation, migration and capillary-like tube formation and less permeability and tumor necrosis factor-a-induced apoptosis. In keeping with a vascular protective effect, adropin stimulated Akt Ser(473) and endothelial nitric oxide (NO) synthase Ser(1177) phosphorylation. The former was abrogated in the presence of the phosphatidylinositol 3-kinase inhibitor LY294002, whereas the latter was attenuated by LY294002 and by mitogen-activated protein kinase kinase 1 inhibition with PD98059. Together, these findings suggest that adropin regulates NO bioavailability and events via the phosphatidylinositol 3-kinase-Akt and extracellular signal regulated kinase 1/2 signaling pathways. Adropin markedly upregulated vascular endothelial growth factor receptor-2 (VEGFR2) transcript and protein levels, and in VEGFR2-silenced endothelial cells, adropin failed to induce phosphorylation of endothelial NO synthase, Akt, and extracellular signal regulated kinase 1/2, supporting VEGFR2 as an upstream target of adropin-mediated endothelial NO synthase activation. Last, adropin improved murine limb perfusion and elevated capillary density following induction of hindlimb ischemia.
We report a potential endothelial protective role of adropin that is likely mediated via upregulation of endothelial NO synthase expression through the VEGFR2-phosphatidylinositol 3-kinase-Akt and VEGFR2-extracellular signal regulated kinase 1/2 pathways. Adropin represents a novel target to limit diseases characterized by endothelial dysfunction in addition to its favorable metabolic profile.

Adropin stimulates neovascularization in vivo in an eNOS-dependent manner. A through D, Balb/c mice were subjected to unilateral (left) hindlimb ischemia before administration of the adropin or null plasmid. A, Quantification of blood flow recovery after hindlimb ischemia surgery; n=4 to 7; *P < 0.05 vs corresponding values for null group. B, Representative laser Doppler images with the highest perfusion level shown in red. C, Quantification of capillary density in gastrocnemius sections as discerned by rhodamine-conjugated isolectin-B4-positive areas; n=3, *P < 0.05 vs null group. D, Representative Western blot analyses for VEGFR2, phospho-eNOS, phospho-Akt, and phospho-ERK1/2 from ischemic gastrocnemius muscles harvested 28 days after hindlimb ischemia surgery. E, Quantification of blood flow recovery after hindlimb ischemia surgery in Nos3tm1Unc/J mice treated with either the adropin or the null plasmid; n=5.
Lovren F. et al. Circulation 2010 Sep 14;122(11 Suppl):S185-92
(Note: this publication uses the bioactive peptide, Adropin, from Phoenix)
|

|
Recent studies have suggested that a higher body mass index (BMI) is associated with an improved prognosis in heart failure (HF). Adropin is a recently identified protein that has been implicated in the maintenance of energy homeostasis. In the present study, we investigated plasma adropin levels in patients with HF and evaluated the relationship between the levels and the severity of HF.
METHODS AND RESULTS: The study group comprised 56 patients with HF and 20 control subjects, who were divided into 4 subgroups according to New York Heart Association (NYHA) functional classification. Plasma levels of adropin, brain natriuretic peptide (BNP) and cardiac hemodynamics were determined. Plasma adropin levels were significantly increased according to the severity of NYHA class in the patients with HF; control: 6.0 ± 0.3; NYHA functional Class II: 7.6 ± 0.4; NYHA functional Class III: 9.8 ± 0.5; NYHA functional Class IV: 12.4 ± 0.6 ng/mL (p < 0.01). Similarly, plasma BNP levels were significantly increased in accordance with the NYHA class. Plasma adropin levels were correlated positively with BNP (r=0.723, p< 0.001), interleukin-6 (IL-6) (r=0.326, p=0.007) and body mass index (BMI) (r=0.295, p=0.014), and negatively with left ventricular ejection fraction (LVEF) (r=-0.710, p< 0.001).
CONCLUSION: Plasma adropin levels were significantly increased according to the severity of HF, and BNP and BMI had independent impact on the plasma adropin level. These findings suggest that the augmented release of adropin may be involved in the pathogenesis of HF and further study is necessary to explain the precise role of adropin in HF.
Lian W, et al. Intern Med. 2011;50(15):1523-7. Epub 2011 Aug 1.
|

|
Recent studies have suggested that a higher body mass index (BMI) is associated with an improved prognosis in heart failure (HF). Adropin is a recently identified protein that has been implicated in the maintenance of energy homeostasis. In the present study, we investigated plasma adropin levels in patients with HF and evaluated the relationship between the levels and the severity of HF.
METHODS AND RESULTS: The study group comprised 56 patients with HF and 20 control subjects, who were divided into 4 subgroups according to New York Heart Association (NYHA) functional classification. Plasma levels of adropin, brain natriuretic peptide (BNP) and cardiac hemodynamics were determined. Plasma adropin levels were significantly increased according to the severity of NYHA class in the patients with HF; control: 6.0 ± 0.3; NYHA functional Class II: 7.6 ± 0.4; NYHA functional Class III: 9.8 ± 0.5; NYHA functional Class IV: 12.4 ± 0.6 ng/mL (p < 0.01). Similarly, plasma BNP levels were significantly increased in accordance with the NYHA class. Plasma adropin levels were correlated positively with BNP (r=0.723, p< 0.001), interleukin-6 (IL-6) (r=0.326, p=0.007) and body mass index (BMI) (r=0.295, p=0.014), and negatively with left ventricular ejection fraction (LVEF) (r=-0.710, p< 0.001).
CONCLUSION: Plasma adropin levels were significantly increased according to the severity of HF, and BNP and BMI had independent impact on the plasma adropin level. These findings suggest that the augmented release of adropin may be involved in the pathogenesis of HF and further study is necessary to explain the precise role of adropin in HF.
No. of Sample (N) = 56 Log transformation was used to normalize the distribution of plasma levels of BNP and TNF-alpha. The parameters listed were variables associated with plasma adropin levels at the p < 0.1 level in the simple linear regression analysis (Figure 2).
No. of Sample (N) = 56 The parameters listed were variables associated with plasma adropin levels at the p < 0.1 level in the simple linear regression analysis.

Figure 1. Plasma adropin levels in the patients with heart
failure according to New York Heart Association (NYHA)
functional class (p-value for Kruskal-Wallis H test, p<0.001).
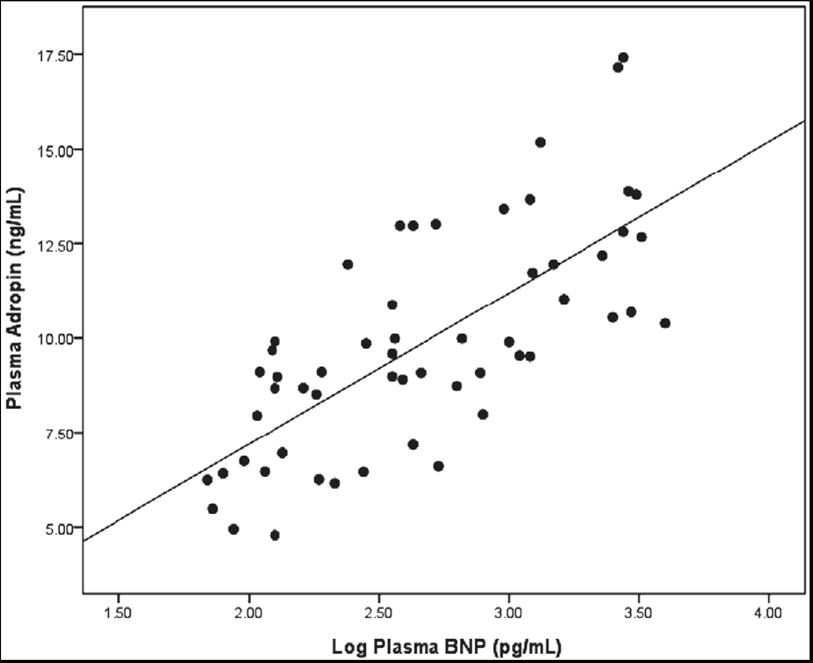
Figure 2. Relationship of plasma adropin level with plasma
level of brain natriuretic peptide (BNP) in patients with heart
failure (r=0.728, p< 0.001). Log transformation was used to
normalize the distribution of the plasma levels of BNP (n=56).
Lian W, et al. Intern Med. 2011;50(15):1523-7. Epub 2011 Aug 1.
|
|
|
|
%032-35%
|
|
|


