|
|
|
Phoenixin |
Bioactive neuropeptide from C4orf52 |
Phoenixin (PNX) is a recently discovered neuropeptide shown to be involved in regulating the reproductive system, anxiety-related behaviors and pain though its receptor is still unknown. PNX-14, one of the endogenous active isoforms, is reported to regulate gonadotropin releasing hormone (GnRH) receptor expression and GnRH secretion. Because GnRH system is thought to be involved in the regulation of learning and memory processes, we hypothesized that PNX-14 might be mediate learning and memory. Here, we investigated the effects of PNX-14 in memory processes, using novel object recognition (NOR) and object location recognition (OLR) tasks. Our results revealed that intracerebroventricular (i.c.v.) injection of PNX-14 (25nmol) immediately after training not only facilitated memory formation, but also prolonged memory retention in both tasks. The memory-enhancing effects of PNX-14 were also seen when it was infused into the hippocampus. Moreover, these memory-improving effects of PNX-14 could be blocked by a GnRH receptor antagonist (Cetrorelix). The memory-improving effects of PNX-14 were not related to any effects on locomotor activity. Additionally, the results suggested that i.c.v. injection of PNX-14 mitigate the memory impairment induced by the amyloid-β1-42 (Aβ1-42) peptide and scopolamine. The present results indicate that PNX-14 facilitates memory formation and prolongs memory retention through activation of the GnRH receptor, and mitigates the memory-impairing effects of Aβ1-42 and scopolamine, suggesting that PNX-14 may be effective as a drug for enhancing memory and treating Alzheimer's disease.
Jiang JH, He Z, Peng YL et al., Brain Res. 2015 Oct 23. pii: S0006-8993(15)00792-1. doi: 10.1016/j.brainres.2015.10.030. [Epub ahead of print]
The hypothalamus regulates a number of autonomic functions essential for homeostasis; therefore, investigations concerning hypothalamic neuropeptides and their functions and distribution are of great importance in contemporary neuroscience. Recently, novel regulatory factors expressed in the hypothalamus have been discovered, of which nesfatin-1 and phoenixin (PNX), show intriguing similarities in their brain distributions. There are currently few studies characterizing PNX expression, so it is imperative to accurately trace its localization, with particular attention to the hypothalamic nuclei and nesfatin-1 co-expression. Using fluorescence and classical immunohistochemical stainings on adult rat brain, we visualized the potential co-expression of nesfatin-1 and PNX immunoreactive cells. We have demonstrated a distinct PNX-immunoreactivity in 21-32% of cells in the arcuate nucleus, paraventricular nucleus, ventromedial and lateral hypothalamus. Nesfatin-1 expression reached 45-68% of all neurons in the same sites, while co-expression was strikingly seen in the vast majority (70-86%) of PNX-immunoreactive neurons in the rat hypothalamic nuclei. Our results demonstrate for the first time, a wide distribution of PNX in the hypothalamus which could implicate a potential functional relationship with nesfatin-1, possibly in the regulation of the hypothalamic-pituitary-gonadal axis or other autonomic functions, which require further study.
Palasz A, Rojczyk E, Bogus K et al., Neurosci Lett. 2015 Feb 28. pii: S0304-3940(15)00175-5. doi: 10.1016/j.neulet.2015.02.060. [Epub ahead of print]
Phoenixin is an amidated neuropeptide, which is widely distributed in brain and periphery regions and is known for its key role in reproduction. Phoenixin-14 (PNX-14), one of the endogenous active isoforms, was reported to regulate pituitary gonadotrophin secretion by increasing the expression of the GnRH receptor mRNA. Studies showed that GnRH could regulate brain responses to anxiety. However, the role of PNX-14 in anxiety was largely unclear. Here, we investigated that the effects of PNX-14 in anxiety-related behavior in adult mice via the open field and elevated plus maze. PNX-14 was administered intracerebroventricularly (i.c.v.) in different doses (5, 10, 25 and 50nmol), and dose-dependently induced anxiolytic effects. Then this anxiolytic action was presented after PNX-14 injected into the anterior hypothalamic area (AHA), while PNX-14 infused into the amygdala did not exert anxiolytic effects. GnRH receptor antagonist (Cetrorelix) could significantly antagonize the anxiolytic effects of PNX-14, while Atosiban, a competitive vasopressin/oxytocin receptor antagonist could not. Moreover, PNX-14 could significantly lower the core temperature and Cetrorelix could block this effect of PNX-14. Additionally, the AHA infusion of PNX-14 (5nmol) increased the expression level of the GnRH mRNA in the hypothalamus and plasma concentrations of GnRH. Similarly, i.c.v. injection of PNX-20 also reduced the core temperature and exerted anxiolytic effects. Taken together, centrally injected PNX-14 generates anxiolytic effects in mice, via the activation of the AHA GnRH system.
Phoenixin-14 amide, herein referred to as phoenixin, is a newly identified peptide from the rat brain. Using a previously characterized rabbit polyclonal antiserum against phoenixin, enzyme-immunoassay detected a high level (>4.5 ng/g tissue) of phoenixin-immunoreactivity (irPNX) in the rat spinal cords. Immunohistochemical studies revealed irPNX in networks of cell processes in the superficial dorsal horn, spinal trigeminal tract and nucleus of the solitary tract; and in a population of dorsal root, trigeminal and nodose ganglion cells. The pattern of distribution of irPNX in the superficial layers of the dorsal horn was similar to that of substance P immunoreactivity (irSP). Double-labeling the dorsal root ganglion sections showed that irPNX and irSP express in different populations of ganglion cells. In awake mice, intrathecal injection of phoenixin (1 or 5 μg) did not significantly affect the tail-flick latency as compared to that in animals injected with artificial cerebrospinal fluid (aCSF). Intrathecal administration of phoenixin (0.5, 1.25 or 2.5 μg) significantly reduced the number of writhes elicited by intraperitoneal injection of acetic acid (0.6%, 0.3 ml/30 g) as compared to that in mice injected with aCSF. While not affecting the tail-flick latency, phoenixin antiserum (1:100) injected intrathecally 10 min prior to the intraperitoneal injection of acetic acid significantly increased the number of writhes as compared to mice pre-treated with normal rabbit serum. Intrathecal injection of non-amidated phoenixin (2.5 μg) did not significantly alter the number of writhes evoked by acetic acid. Our result shows that phoenixin is expressed in sensory neurons of the dorsal root, nodose and trigeminal ganglia, the amidated peptide is bioactive, and exogenously administered phoenixin may preferentially suppress visceral as opposed to thermal pain.
Lyu RM, Huang XF, Zhang Y et al., Neuroscience. 2013 Oct 10;250:622-31. doi: 10.1016/j.neuroscience.2013.07.057. Epub 2013 Jul 31.
Normal anterior pituitary function is essential for fertility. Release from the gland of the reproductive hormones luteinising hormone and follicle-stimulating hormone is regulated primarily by hypothalamically-derived gonadotrophin-releasing hormone (GnRH), although other releasing factors (RF) have been postulated to exist. Using a bioinformatic approach, we have identified a novel peptide, phoenixin, that regulates pituitary gonadotrophin secretion by modulating the expression of the GnRH receptor, an action with physiologically relevant consequences. Compromise of phoenixin in vivo using small interfering RNA resulted in the delayed appearance of oestrus and a reduction in GnRH receptor expression in the pituitary. Phoenixin may represent a new class of hypothalamically-derived pituitary priming factors that sensitise the pituitary to the action of other RFs, rather than directly stimulating the fusion of secretary vesicles to pituitary membranes.
Yosten GL et al., J Neuroendocrinol. 2013 Feb;25(2):206-15. doi: 10.1111/j.1365-2826.2012.02381.x.
Cellular processes often depend on stable physical associations between proteins. Despite recent progress, knowledge of the composition of human protein complexes remains limited. To close this gap, we applied an integrative global proteomic profiling approach, based on chromatographic separation of cultured human cell extracts into more than one thousand biochemical fractions that were subsequently analyzed by quantitative tandem mass spectrometry, to systematically identify a network of 13,993 high-confidence physical interactions among 3,006 stably associated soluble human proteins. Most of the 622 putative protein complexes we report are linked to core biological processes and encompass both candidate disease genes and unannotated proteins to inform on mechanism. Strikingly, whereas larger multiprotein assemblies tend to be more extensively annotated and evolutionarily conserved, human protein complexes with five or fewer subunits are far more likely to be functionally unannotated or restricted to vertebrates, suggesting more recent functional innovations.
Havugimana PC, Hart GT, Nepusz T et al., Cell. 2012 Aug 31;150(5):1068-81. doi: 10.1016/j.cell.2012.08.011.
SUMMARY
Bioinformatic analysis of evolutionarily conserved sequences in the hypothetical proteins human LOC389203 and rat LOC501923 predict the possibility of peptides which we name Phoenixin-20 amide, Phoenixin-15 (Phoenixin-14-Gly), and Phoenixin-14 amide. Based on the conserved sequence, these peptides were synthesized and rabbit polyclonal antisera against them were generated. After purification of heart and hypothalamus homogenate extracts from both bovine and rat tissues, a HPLC fraction showed an above ng/ml concentration of immunoreactive Phoenixin (ir-Phoenixin) in the immunoassays and indicated the Phoenixins were present in tissues. From HPLC purification and analysis, the natural peptides corresponding to the predicted Phoenixin sequence were revealed. The sequences were also confirmed by mass-spectrometry analysis and corresponding profiles of synthetic peptides. In summary, high levels of Phoenixins for the hypothalamus and heart homogenates can be quantified by immunoassay. Localization of ir-Phoenixin cells also has been identified with Immunohistochemical (IHC) staining on various tissues, including brain. In vivo and In vitro functional assays of Phoenixin have been under investigation. The Phoenixin peptide hormones have been discovered in brain/heart tissues and found to have diverse cellular function and physiological activities.
INTRODUCTION
There is considerable interest in the discovery of new endogenous ligands such as peptides that modulate the homeostasis of multicellular organisms. Based on bioinformatic analysis of signal peptide and proteolytic processing sites, we predicted the existence of several peptides in the uncharacterized protein LOC389203 and LOC501923. As a first step towards realizing the function of these peptides, we first isolated the peptides and generated antibodies against the putative peptides. The identified peptides, named Phoenixin-20 amide, Phoenixin-15 (Phoenixin-14-Gly), and Phoenixin-14 amide are found in the heart, and certain regions in the brain.
CONCLUSIONS
• The peptides, Phoenixin-20 amide, Phoenixin-15, and Phoenixin-14 amide have been identified in brain and heart tissues.
• The amounts of Phoenixin-20 amide and Phoenixin-14 amide are about 150 pg/mg protein both in the rat heart and bovine hypothalamus.
• Phoenixin-14 amide appears to be able to compete with Phoenixin-20 amide for binding to pituitary cells.
• The stimulation of cAMP production for pituitary cells implies those peptides might have an important extracellular function and physiological activities.
Rong-Ming Lyu, Xiang-Qun Chen, Qing Tian, Oliver Jahraus, Nae J. Dun and Jaw-Kang Chang: Isolation, identification, and distribution of Phoenixin, Presented at the 22nd American Peptide Symposium, 2011.
(Manuscript has been submitted for publication in "proceedings of
the 22nd American Peptide Symposium".)
Peptides and assay kits will be available exclusively from Phoenix Pharmaceuticals, due to US patent pending.
FMRFamide and the related tetrapeptide FLRFamide are highly excitatory in molluscan non-cardiac smooth muscle. They are also exceptionally excitatory in the atrium and internally perfused ventricle of Busycon canaliculatum. These two peptides, usually thought of as classic molluscan cardio-acceleratory agents are in fact simply two members of a large and ever growing superfamily, the RFamide family, whose phylogenetic distribution has been so elegantly mapped by Walker. Members of this family, often with extended peptide chains (e.g. penta, hepta and decapeptides), stretch in their known distribution from the cnidaria to the chordates. The effects of some of the members of this superfamily (FMRFamide. FLRFamide, YMRFamide, TNRNFLRFamide, SDPFLRFamide, LMS) were examined. The neuropeptides were found to be very potent at very low concentrations (10(-9) M) in the ventricle of both Buccinium and Busycon. Other neuropeptides (HFMRdFamide, SCPb, NLERFamide and pEGRFamide) were found to be without any effect. The Ca2+ dependency of these neuropeptides was also tested. The peptides appear to induce contraction of the ventricles by release of Ca2+ from internal pools. The neuropeptides appear to stimulate contraction in these cardiac muscles through a completely different pathway to Serotonin (the main excitatory neurotransmitter for the cardiac muscle). When the peptides were applied together with Serotonin an additive effect was observed clearly indicating the release of Ca2+ through different pathways. The nature of the RFamide receptor was also tested. It appears that the RFamide neuropeptides mobilize the 2nd messenger IP3 (Inositol trisphosphate), since the IP3 blocker Neomycin Sulphate inhibited the response of the neuropeptides.
Moulis A Acta Biol Hung. 2004;55(1-4):335-41.
MALDI-ToF MS (matrix-assisted laser desorption/ionization time of flight mass spectrometry) has become a fast, reliable and sensitive technique for the identification of neuropeptides in biological tissues. Here, we applied this technique to identified neurons of the cardioregulatory network in the snail Lymnaea that express the FMRFamide gene. This enabled us to study the complex processing of the FMRFamide gene at the level of single identified neurons. In the CNS of Lymnaea, FMRFamide-like and additional peptides are encoded by a common, multiexon gene. Alternate mRNA splicing of the FMRFamide gene leads to the production of two different mRNAs. Type 1 mRNA (exon II) encodes for the tetrapeptides (FLRF/FMRFamide), whereas Type 2 (exons III-V) encodes for the heptapeptides (SDPFLRFamide/GDPFLRFamide). Previous in situ hybridization and immunocytochemical studies indicated that these two transcripts are expressed in the CNS neurons of Lymnaea in a differential and mutually exclusive manner. Two single identified neurons of the cardiorespiratory network, the Ehe neuron and the visceral white interneuron (VWI), were known to express the FMRFamide gene (Ehe, type 1 mRNA; VWI, type 2 mRNA). MALDI-TOF MS analysis of these neurons and other neurons expressing the FMRFamide gene confirmed the mutually exclusive expression of the distinct sets of peptides encoded on the two transcripts and revealed the pattern of post-translational processing of both protein precursors. From the gene sequence it was predicted that 16 final peptide products from the two precursor proteins could possibly exist. We showed that most of these peptides were indeed present in the identified neurons (13) while others were not (three), suggesting that not all of the potential cleavage sites within the two precursors are utilized. In this way, the neuronal expression of the full range of the peptide products resulting from alternative mRNA splicing was revealed for the first time.
Worster BM, Yeoman MS, Benjamin PR. Eur J Neurosci. 1998 Nov;10(11):3498-507.
The effect of several synthetic FMRF-like peptides in hemodynamics and tonus of isolated rat vessels was studied. Peptide pGlu-Asp-Pro-Phe-Leu-Arg-Phe-NH2 and its C-terminal fragment Arg-Phe-NH2 induced i.v. short-term increase in arterial blood pressure and heart rate in narcotized rats. Benzpgexonium could reduce both effects of the drug. The peptide effect on blood pressure could be neutralized by prazosin; tachycardia could be prevented by propranolol. The studied peptide and its C-end tetra-, penta- and dipeptide produced relaxation of isolated rat aorta toned by prostaglandin F2 alpha. It is supposed that hypertensive effect of FMRF-like peptides is realized through central mechanisms.
Zhukovskii SV, Korobov NV, Deigin VI et al., Biull Vsesoiuznogo Kardiol Nauchn Tsentra AMN SSSR. 1989;12(1):45-7.
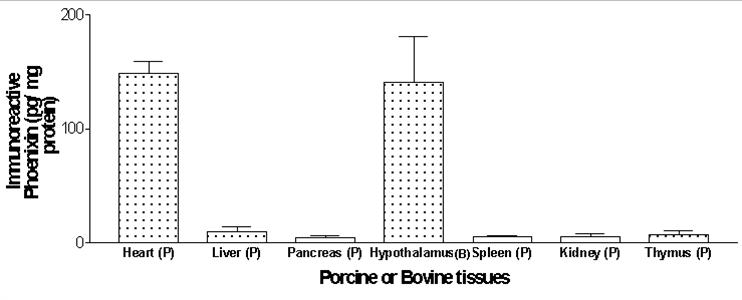
The level of immunoreactive Phoenixin in porcine (P) or bovine (B) tissues were measured by using a specific RIA kit that recognizes both Phoenixin-20 amide and Phoenixin-14 amide.
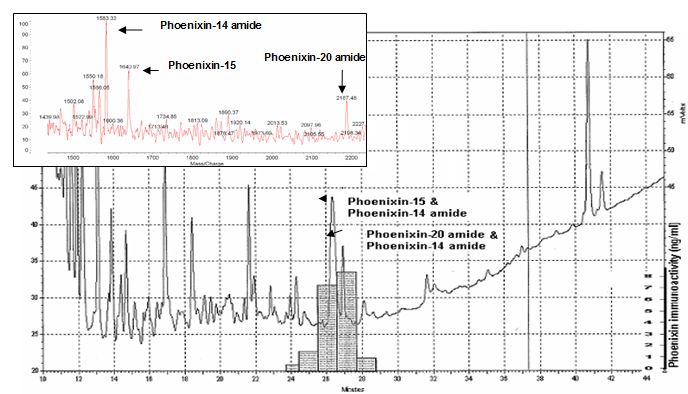
HPLC profile and immunoreactivity of isolated peptides- In the heart homogenate extracts, the major peaks of phoenixin immunoreactive fractions were from the elutes at 26 and 27 min of the 1st HPLC column. The major immunoreactive fraction at 27 min of 1st HPLC contains peptide ions at MW 1583, 1641 and 2187 which were corresponding to the theoretical molecular weight of the peptides, Phoenixin-14 amide, Phoenixin-15 and Phoenixin-20 amide. In addition, the synthetic peptides, Phoenixin-14 amide, Phoenixin 15 and Phoenixin-20 amide eluted from the HPLC at 27 min and showed the same peak positions around the MW of 1583, 1641 and 2184 in mass spectrometry.
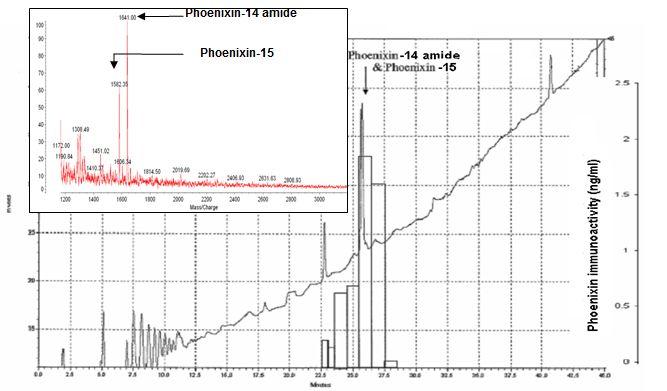
The 2nd HPLC profile, immunoreactivity and Mass spectrometry to identify the isolated peptides- The fractions with highest immunoreactivity in 2nd HPLC were from the peak between 26 min and 27 min. The fraction collected at 26 min, the isolated peptide identified by Mass spectrometer were at MW 1582.3 and 1641 which represent Phoenixin-14 amide and Phoenixin-15.
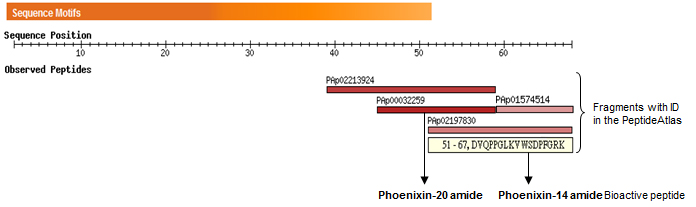
The gene of C4orf52 encoded a 67 residues protein. Two bioactive neuropeptides are generated from this encoded protein.

Alignment of amino acid sequences of rat hypothetical protein LOC501923 and human protein LOC389203 isoform 2.
Alignment of amino acid sequences of homologous proteins from different species show high evolution sequence conservation. Based on potential proteolytic cleavage sites, three C-terminal hypothetical peptides are predicted: Phoenixin-20 amide, Phoenixin15 (Phoenixin-14-Gly), and Phoenixin-14 amide.
Phoenixin-immunoreactive cell bodies are detected in the caudate putamen (CPu) in Figure (A); fine cell processes can also be seen in the CPu in Figure (B); amygdala Figure (C), and periventricular nucleus (Pe) in Figure (D). Scale bar: (A), 100 µm; (B), (C) and (D), 50 µm.
| Peptides |
%Cross-Reactivity |
| Phoenixin-14 amide |
100 |
| Phoenixin-20 amide |
100 |
| Phoenixin-15
(Phoenixin-14-Gly) |
0 |
| [pGlu1]-Phoenixin-25-Gly (Porcine) |
0 |
| Adrenomedullin (1-52) (H) |
0 |
Angiotensin I (H,R,M,P,C,Rabbit) |
0 |
| Angiotensin II (H,R,M,P,C) |
0 |
| Apelin-12 (H,R,M,B) |
0 |
| ANP-alpha (1-28)(H,P,O,C) |
0 |
| Bradykinin (H,R,M) |
0 |
| BNP-32 (H) |
0 |
| LH-RH / GnRH (H,R,M,P) |
0 |
| NPY (H) |
0 |
| Orexin A (H,B,M,R) |
0 |
| Somatostatin |
0 |
| TLQP-21 (H) |
0 |
| Cross-Reactivity |
| Phoenixin-20 Amide (H,R,M,P,B) |
100 |
| Phoenixin-14 Amide (H,R,M,P,B,C) |
50 |
| Phoenixin-15 (H,R,M,P,B,C) |
0 |
| Sensitivity |
Minimum Detectable Concentration: 0.3ng/ml |
| Range |
100-12800pg/ml
|
| Lowest Detection Limit |
00pg/ml |
|
|
|
%phoenixin%
|
|
|


