|
|
| 
| Examination of hypothalamic mRNA has led to the discovery of a small protein termed Beacon (Collier et al, 2000). ICV administration of Beacon increased NPY expression and stimulated food intake in a dose-dependent manner. Simultaneous infusion of Beacon with NPY significantly potentiated orexigenic responses and results in rapid body weigh gain. Beacon has been found through immunohistochemical methods to be present in a wide variety of brain areas suggesting that in addition to a role in feeding, it may possess a multitude of biological activities associated with the hypothalamic-pituitary axis (Brailou et al, 2000).
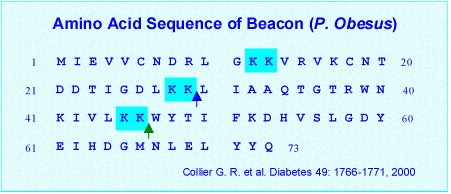
Collier G. R. , et al. A Novel gene involved in the regulation of energy balance. Diabetes 49:1766-1771, 2000.
Brailoiu GC, Dun SL, Yang J, Chang JK, Castellino S, Dun NJ. Beacon-like immunoreactivity in the hypothalamus of Sprague-Dawley rats. Neurosci Lett 2002 Jan 14;317(3):166-8
Walder K, Ziv E, Kalman R, Whitecross K, Shafrir E, Zimmet P, Collier GR. Elevated hypothalamic beacon gene expression in Psammomys obesus prone to develop obesity and type 2 diabetes. Int J Obes Relat Metab Disord 2002 May;26(5):605-9
|

Beacon is a novel peptide
isolated from the hypothalamus of Israeli sand rat. In the present
study, we determined the distribution of beacon in the rat brain
using immunohistochemical approachwith a polyclonal antiserum
directed against the synthetic C-terminal peptide fragment (47–73).
The hypothalamus represented the major site of beacon-immunoreactive
(IR) cell bodies that were concentrated in the paraventricular
nucleus (PVN) and the supraoptic nucleus (SON). Additional immunostained
cells were found in the septum, bed nucleus of the stria terminalis,
subfornical organ and subcommissural organ. Beacon- IR fibers
were seen with high density in the internal layer of the median
eminence and low to moderate density in the external layer.
Significant beacon-IR fibers were also seen in the nucleus of
the solitary tract and lateral reticular formation. The beacon
neurons found in the PVN were further characterized by double
label immunohistochemistry. Several beacon- IR neurons that
resided in the medial PVN were shown to coexpress corticotrophinreleasing
hormone (CRH) and most labeled beacon fibers in the external
layer of median eminence coexist with CRH. The topographical
distribution of beacon-IR in the brain suggests multiple biological
activities for beacon in addition to its proposed roles in modulating
feeding behaviors and pituitary hormone release.
Wang F., et
al. Peptides, 27 ( 2006 ) 165–171
Beacon gene is overexpressed
in obese rats, and beacon was found to stimulate food intake.
Evidence has been recently provided that beacon is also expressed
in the endocrine glands of normal rats, including adrenal cortex,
of which it appears to regulate secretory activity. To further
characterize the role of beacon in the rat adrenals, we investigated
the level of beacon expression in the adrenal zona glomerulosa
(ZG), zona fasciculata-reticularis (ZF/R) and medulla (AM),
and the in vitro secretory responses to beacon[47-73] (hereinafter,
beacon) of adrenocortical and adrenomedullary tissues. Real-time
polymerase chain reaction revealed similar high levels of beacon
mRNA in the ZG and ZF/R, and significantly lower (-80%) levels
in AM. Immunocytochemistry showed that the distribution of beacon
protein followed that of beacon mRNA. Quantitative high pressure
liquid chromatography demonstrated that beacon (5x10(-7) M)
reduced by about 56% the in vitro total steroid-hormone production
from ZG and ZF/R tissues, without affecting catecholamine secretion
from AM specimens. The beacon-induced lowering in the secretory
activity of adrenal cortex depended on similar reductions (from
50-64%) in the production of the main adrenocortical hormones
(pregnenolone, progesterone, 11-deoxycorticosterone, corticosterone,
18-hydroxy-corticosterone and aldosterone), thereby suggesting
an inhibitory action of beacon in the early step of steroidogenesis
(i.e. the conversion of cholesterol to pregnenolone). The hypothesis
is advanced that beacon is to be considered an autocrine-paracrine
negative regulator of mineralo- and glucocorticoid synthesis
in the rat adrenal gland.
Rucinski M,
et al. Int J Mol Med. 2005 Jul;16(1):35-40
OBJECTIVE: Beacon gene expression
is elevated in the hypothalamus of the Israeli sand rat (Psammomys
obesus), an animal that is used as a polygenic animal model
of obesity and NIDDM. We performed studies aimed at investigating
the expression of beacon mRNA and protein in pancreatic islets
of the rat and the possible beacon protein effects on insulin
secretion.
METHODS: Rat pancreatic islets were isolated by the
collagenase digestion technique. Beacon mRNA expression was
demonstrated in isolated islets using RT-PCR and beacon-like
immunoreactivity using immunocytochemistry (ICC) on a sections
of Bouin-fixed pancreas. Isolated islets were incubated with
1-100 nmol/L beacon (47-73) protein in normoglycemic medium.
Adult female rats were subcutaneously injected with beacon (47-73)
at doses 0.35 or 0.7 nmol/100 g body weight and killed after
30 and 60 minutes.
RESULTS: RT-PCR results indicate the presence
of beacon mRNA in isolated rat pancreatic islets. Beacon-like
immunoreactivity is present in all cell types of the Langerhans
islet. Beacon inhibits insulin secretion from isolated islets.
In contrast, a bolus administration of beacon at a lower dose
notably stimulates blood insulin concentration at 30 and 60
minutes of the experiment while the higher dose does not change
insulinemia. None of the treatment had an effect on blood glucose
concentration.
CONCLUSION: This study demonstrated the presence
of beacon mRNA in isolated rat islets as well as a direct inhibitory
effect of beacon protein on insulin secretion by isolated rat
pancreatic islets. The data obtained suggest that beacon may
be involved in physiologic regulation of insulin secretion.
Nowak KW, et
al. Pancreas. 2004 Aug;29(2):99-103
Evidence has been recently
provided that beacon, an ubiquitin-like protein overexpressed
in the hypothalamus of Israeli sand rat, is also expressed in
several endocrine glands of the Wistar rat, including adrenal
cortex. Moreover, it has been shown that the in vivo administration
of beacon[47-73] (hereinafter, beacon) evokes within 60 min
a marked decrease in the plasma concentrations of ACTH and corticosterone.
Hence, we have investigated the effect of beacon (4x10(-9) or
4x10(-7) M) on the secretion and growth of cultured rat and
human zona fasciculata/reticularis (ZF/R) cells. Reverse transcription-polymerase
chain reaction detected beacon mRNA in all human adrenal cortexes
examined. A 3-h exposure to beacon was ineffective, but prolonged
(24 and 96 h) exposures significantly lowered basal corticosterone
and cortisol secretion from cultured rat and human ZF/R cells,
respectively. Moreover, beacon (4x10(-7) M) counteracted the
secretagogue action of 10(-8) M ACTH on cultured cells. The
96-h exposure to beacon concentration-dependently decreased
basal proliferation rate of cultured cells, without inducing
significant changes in the number of apoptotic and necrotic
cells. Beacon (4x10(-7) M) significantly inhibited the proliferogenic
effect of 10(-8) M adrenomedullin. In light of the involvement
of ubiquitin-like proteins in the control of cell cycle and
protein sorting and degradation, the hypothesis is advanced
that the inhibitory effect of beacon on the secretion and growth
of cultured rat ZF/R cells may be connected to its stimulating
effect on proteolysis of steroidogenic enzymes and proteins
involved in cell replication.
Ziolkowska A,
et al. Int J Mol Med. 2004 Sep;14(3):457-61
Distribution of the novel
peptide beacon in the hypothalamus of Sprague-Dawley rats was
examined by immunohistochemical methods. Beacon-immunoreactive
(irBC) neurons were found in the paraventricular, supraoptic,
and accessory neurosecretory nuclei, and intensely labeled fibers
in the median eminence and infundibulo-pituitary stalk. Scattered
cells and/or fibers were noted in the suprachiasmatic nucleus,
arcuate nucleus, retrochiasmatic area, lateral and medial preoptic
area, as well as anterior and lateral hypothalamic area. The
wide distribution of irBC in the hypothalamus of Sprague-Dawley
rats suggests that the peptide may influence, in addition to
a proposed role in feeding, a multitude of biological activities
associated with the hypothalamic-pituitary axis.
Brailoiu GC,
Dun SL, Yang J, Chang JK, Castellino S, Dun NJ. Neurosci Lett
2002 Jan 14;317(3):166-8
 

Coronal sections illustrating the distribution of beacon-containing cell bodies and nerve fibers in the dorsal lateral septal nucleus (LSD) (A) and the medial posterior region of the bed nucleus of the stria terminalis (BNSTMP) (B). Scale bar = 200 mm. Wang F., et al. Peptides, 27 (2006) (165–171) [Primary antibody: Rabbit Anti Beacon (47-73) Antiserum, H-072-50].

Coronal sections showing neurons containing beacon-IR in the hypothalamus. The main population of beacon-IR cells were noted in the PVN (A) and SON (B). In the PVN, beacon-positive cell bodies and nerve fibers were found both in the magnocellular and parvocellular part of the nucleus (A). Quite a number of beacon-IR neurons and nerve fibers were detected in the SCN (C) and SOR (D).
Abbreviation: dp, dorsal parvocellular; mp, medial parvocellular; F, fornix; OX, optic chiasm; 3V, third ventricle. Scale bar = 200 mm.
Wang F., et al. Peptides, 27 ( 2006 ) 165–171

Coronal sections illustrating beacon-stained nerve fibers in the nucleus of the solitary tract (NTS) (A and B) and the lateral reticular nucleus (LRt) (C). Abbreviation: AP, area postrema; cc, central canal; ncom, commissural nucleus of NTS; mNTS, medial subnucleus of NTS. Scale bar = 200 mm.
Wang F., et al. Peptides, 27 ( 2006 ) 165–171



Coronal sections illustrating the localization of beacon-IR in circumventricular organs including SFO (A and C), ME (B and D) and SCO (C and F). A group of intensely stained beacon-positive cell bodies was detected in the lateral margin of the SFO (A and C). In the ME, most Beacon-IR fibers were seen in the internal layer (B and D). In the SCO, both the ependymal (E) and hypendymal (H) cells are beacon-IR. The arrow shows the blood vessel between the hypendymal cells and basal processes of the ependymal cells. Abbreviation: LV, lateral ventricle; 3V, third ventricle; PC, posterior commissure. Scale bar = 100 mm.
Wang F., et al. Peptides, 27 ( 2006 ) 165–171
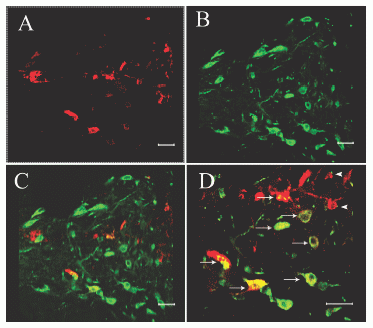
Confocal images of rat paraventricular hypothalamic nucleus double-labeled with beacon-antiserum and CRHantiserum. Section labeled with beacon- (A) and CRH-antiserum(B); and an overlay of the images (A) and (B) (C); (D) is a high magnification of (C). Several beacon-containing neurons were CRH-IR positive (arrows) and some beacon-containing fibers were in close contact with CRH-IR neurons (arrowheads). Scale bar = 40 mm.

Confocal images of rat ME double-labeled with beacon-antiserum and CRH-antiserum. Most beacon-containing fibers in the external layer are CRH-immunoreactive (arrows), but several beacon-IR fibers (arrowhead) are not
CRH-positive. Scale bar = 40 mm.

|
|
|
%beacon%
|
|
|


