| Human Urocortin II, a Selective
Agonist for the Type 2 Corticotropin-Releasing Factor Receptor,
Decreases Feeding and Drinking in the Rat Corticotropin-releasing
factor (CRF) has been hypothesized to modulate consummatory
behavior through the Type 2 CRF (CRF(2)) receptor. However,
behavioral functions subserved by the CRF(2) receptor remain
poorly understood. Recently, human urocortin II (hUcn II), a
selective CRF(2) receptor agonist, was identified. To study
the effects of this neuropeptide on ingestive behavior, we examined
the effects of centrally infused hUcn II (i.c.v. 0, 0.01, 0.1,
1.0, 10.0 &mgr;g) on the microstructure of nose-poke responding
for food and water in nondeprived, male rats. Malaise-inducing
properties of the peptide were monitored using conditioned taste
aversion (CTA) testing. To identify potential sites of action,
central induction of Fos protein expression was examined. hUcn
II dose dependently reduced the quantity and duration of responding
for food and water at doses lower (0.01-1.0 &mgr;g) than
that forming a CTA (10 &mgr;g). Effects were most evident
during hours 4 to 6 of the dark cycle. Meal pattern analysis
showed that hUcn II potently (0.1 &mgr;g) increased the
satiating value of food. Rats ate and drank smaller and shorter
meals without changing meal frequency. Rats also ate more slowly.
hUcn II induced Fos in regions involved in visceral sensory
processing and autonomic/neuroendocrine regulation and resembling
those activated by appetite suppressants. hUcn II is a promising
neuropeptide for investigating the role of the CRF(2) receptor
in ingestive behavior.
Inoue K,et al. J Pharmacol Exp Ther 2003
Apr 1;305(1):385-393
Human Urocortin II (Urocortin Related Peptide,
URP) is identical with Stresscopin Related Peptide (SRP) (6-43)-NH2
(Human).
References:
Lewis, K. et al. Proc. Natl. Acad. Sci. USA 98, 7570-7575,
2001 (June 19)
Hsu, S.Y. & Hsueh, A.J.W. Nature Medicine, 7 605-611,
2001
Review by Jun Yang on June 21, 2001
Human urocortin II, a new CRF-related peptide, displays selective
CRF(2)-mediated action on gastric transit in rats
Human urocortin (hUcn) II is a new member of the corticotropin-releasing
factor (CRF) family that selectively binds to the CRF(2) receptor.
We investigated the CRF receptors involved in mediating the
effects of hUcn II and human/rat CRF (h/rCRF) on gut transit.
Gastric emptying, 4 h after a solid meal, and distal colonic
transit (bead expulsion time) were monitored simultaneously
in conscious rats. CRF antagonists were given subcutaneously
30 min before intravenous injection of peptides or partial restraint
(for 90 min). hUcn II (3 or 10 microg/kg i.v.) inhibited gastric
emptying (by 45% and 55%, respectively) and did not influence
distal colonic transit. The CRF(2) peptide antagonist astressin(2)-B
blocked hUcn II action. h/rCRF, rat Ucn, and restraint delayed
gastric emptying while accelerating distal colonic transit.
The gastric response to intravenous h/rCRF and restraint was
blocked by the CRF(2) antagonist but not by the CRF(1) antagonist
CP-154,526, whereas the colonic response was blocked only by
CP-154,526. None of the CRF antagonists influenced postprandial
gut transit. These data show that intravenous h/rCRF and restraint
stress-induced delayed gastric emptying involve CRF(2) whereas
stimulation of distal colonic transit involves CRF(1). The distinct
profile of hUcn II, only on gastric transit, is linked to its
CRF(2) selectivity.
Million M, et al. Am J Physiol Gastrointest
Liver Physiol 2002 Jan;282(1):G34-40
Differential actions of peripheral corticotropin-releasing
factor (CRF), urocortin II, and urocortin III on gastric emptying
and colonic transit in mice: role of CRF receptor subtypes 1
and 2
Peripheral CRF inhibits gastric emptying and stimulates colonic
motor function in rats. We investigated the role of CRF(1) and
CRF(2) receptors in i.p. CRF-induced alterations of gut transit
in conscious mice using selective CRF(1) and CRF(2) ligands
injected i.p. Gastric emptying 2 h after ingestion of a solid
chow meal and colonic transit (time to expel a bead inserted
into the distal colon) were determined simultaneously. Rat/human
(r/h)CRF, which has CRF(1) > CRF(2) binding affinity, decreased
distal colonic transit time at lower doses (6-12 microg/kg)
than those inhibiting gastric emptying (20-60 microg/kg). Ovine
CRF, a preferential CRF(1) receptor agonist (6-60 microg/kg),
reduced significantly the colonic transit time without altering
gastric emptying, whereas the selective CRF(2) receptor agonists
mouse urocortin II (20-60 microg/kg) and urocortin III (120
microg/kg) inhibited significantly gastric emptying without
modifying colonic transit. The CRF(1)/CRF(2) receptor antagonist,
astressin (30-120 microg/kg), dose dependently prevented r/hCRF
(20 microg/kg)-induced inhibition of gastric emptying and reduction
of colonic transit time. The selective CRF(1) receptor antagonists,
NBI-27914 (C(18)H(20)Cl(4)N(4)C(7)H(8)O(3)S) and CP-154,526
(butyl-[2,5-dimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]e
thylamine) (5-30 mg/kg), dose dependently blocked r/hCRF action
on the colon without influencing the gastric response, whereas
the CRF(2) receptor antagonist, antisauvagine-30 (30-100 microg/kg),
dose dependently abolished r/hCRF-induced delayed gastric emptying
and had no effect on colonic response. These data show that
i.p. r/hCRF-induced opposite actions on upper and lower gut
transit in conscious mice are mediated by different CRF receptor
subtypes: the activation of CRF(1) receptors stimulates colonic
propulsive activity, whereas activation of CRF(2) receptors
inhibits gastric emptying.
Martinez V,et al. J Pharmacol Exp Ther 2002 May;301(2):611-7
Urocortin-Related Peptides Increase Interleukin-6 Output via
Cyclic Adenosine 5'-Monophosphate-Dependent Pathways in A7r5 Aortic
Smooth Muscle Cells Corticotropin-releasing factor receptor
type 2ß, expressed in the rodent cardiovascular system,
is a member of the G protein-coupled receptor family.
This receptor is coupled positively to adenylate cyclase
and is bound preferentially by the urocortin (Ucn)-related
peptides (Uncs): Ucn, Ucn II, and Ucn III. In the present
study, we investigated the effects of Ucns on IL-6
levels in A7r5 aortic smooth muscle cells. In this
cell line, both Ucn and Ucn II induced accumulation
of intracellular cAMP via corticotropin-releasing factor
receptor type 2ß and also caused a significant increase
in IL-6 output levels. The adenylate cyclase inhibitor,
MDL-12330A, inhibited this Ucn- or Ucn II-induced increase
in IL-6 levels. Although H89 (10 µM),
a protein kinase A inhibitor, had no effect on the
increase in IL-6 concentration, bisindolylmaleimide
I (10 nM), a protein kinase C inhibitor,
was found to significantly inhibit IL-6 output levels.
Blockade of Ucn- or Ucn II-induced increases in IL-6
levels by SB203580 (100 nM),
a p38 MAPK inhibitor, suggested that the p38 MAPK pathway
was involved in this regulation. The cAMP-mediated increase
in IL-6 levels was suppressed synergistically by both
bisindolylmaleimide I and SB203580. These findings
demonstrate that both protein kinase C and p38 MAPK
signaling cascades are involved downstream of the
Ucns-cAMP pathway in A7r5 aortic smooth muscle cells.
Kazunori Kageyama and Toshihiro
Suda. Endocrinology 2003, 144(6) 2234-2241
|
| 
|
| Figure 1. Expression and localization
of Ucn III in mouse pancreatic islets and MIN6 cells.
A, Representative electrophoretic analysis of the
RT-PCR products of Ucn, Ucn II, and Ucn III in mouse
pancreas and MIN6 cells. S16 RNA was used as positive
control. -RT, RNA samples were used for PCR without
RT; -cDNA, no RT product added to the PCR. B, Representative
autoradiogram showing the detection of Ucn II and
Ucn III mRNAs by RNase protection assay. Total RNA
isolated from mouse brain stem (for Ucn II), hypothalamus
(for Ucn III), or whole pancreas was hybridized with
antisense probes specific to mouse Ucn II or Ucn III
and mouse glyceraldehyde-3-phosphate dehydrogenase.
Ucn II probe protects a 592-nt band in the brain stem
and the pancreas and Ucn III probe protects a 414-nt
band in the hypothalamus and the pancreas. C, Representative
dark-field photomicrograph showing a mouse pancreas
section probed with mouse Ucn III riboprobe. Ucn III
mRNA-positive signals (silver grain clusters) were
found in the pancreatic islets. D, Bright-field photomicrograph
of panel C with nissl staining showing the islets.
*, Blood vessel. E and F, Representative photomicrographs
showing Ucn III immunostaining in the mouse pancreatic
sections without (E) or with (F) preabsorption of
the anti-Ucn III serum with 30 µM
of mouse Ucn III. Ucn III-positive cells (dark
purple staining) were observed only in the islets.
Scale bar, 50 µm. Chien
Li, et al. Endocrinology Vol. 2003,144(7),
3216-3224 |
| 
|
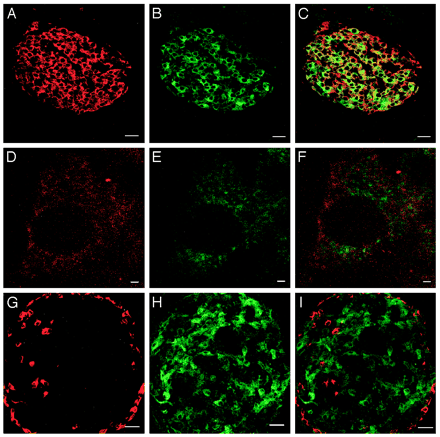
| Figure 2. Colocalization
of Ucn III and insulin in mouse pancreatic islets.
Stacked serial confocal images of insulin (A) and
Ucn III (B) cells in the islet. C, Visualization
of both substances simultaneously showing the majority
of insulin and Ucn III colocalize in the same cells.
High magnification of confocal images showing insulin
(D) and Ucn III (E) in a single ß-cell. F, Combined
image of panels D and E to visualize insulin and
Ucn III simultaneously. Note that little colocalization
is observed. G–I, Stacked serial confocal
images of glucagon (G), Ucn III (H), and combined
image (I) of panels G and H showing no colocalization
of the two substances. Scale bar, 25 µm for
A–C and G–I and 1 µm for D–F.
Chien Li, et al. Endocrinology
Vol. 2003,144(7), 3216-3224 |
Table 2. Effects of Ucn III
and rat/human CRF on glucagon and insulin secretion
from isolated rat islets
|
Ucn III
|
Rat/human CRF
|
| 0.1 |
1 |
10 |
100
(nM) |
1 |
10 |
100
(nM) |
|
| Glucagon |
103.6
± 38a |
157.3
± 18a |
169.7
± 26a1 |
284.1
± 771 |
119.5
± 29a |
95.3
± 15a1 |
281.9
± 791 |
| Insulin |
180.8
± 66d |
296.4
± 51e |
231.8
± 39e |
258.3
± 43e |
132.6
± 24d |
113.3
± 14d |
362.7
± 87e |
|
Data are expressed as percent of control (2.8 mM
glucose). Values in same row with different superscripts
(a, c, d, e) are significantly different
with P < 0.05. 1 Significantly
different from each other with P < 0.05.
Chien Li, et al. Endocrinology
Vol. 2003,144(7), 3216-3224
|
| 
|
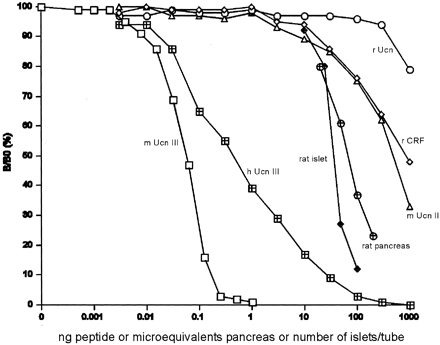
Figure 3. Displacement
of 125I-labeled mUcn III binding to rabbit
anti-mUcn III by mUcn III ( )
and partially purified rat pancreatic ( )
and partially purified rat pancreatic ( )
or isolated rat islet ( )
or isolated rat islet ( )
extract. Rat pancreas or isolated rat islets were
acid-extracted and partially purified using octadecyl
silica cartridges. Human Unc III (h Ucn III, )
extract. Rat pancreas or isolated rat islets were
acid-extracted and partially purified using octadecyl
silica cartridges. Human Unc III (h Ucn III,  )
shows approximately 10% cross-reactivity. Closely
related family peptides including rat Ucn (r Ucn, )
shows approximately 10% cross-reactivity. Closely
related family peptides including rat Ucn (r Ucn,
 ),
rat CRF (r CRF, ),
rat CRF (r CRF,  ),
or mouse Ucn II (m Ucn II, ),
or mouse Ucn II (m Ucn II,  )
showed little or no cross-reactivity. B/B0,
Bound to maximum-bound ratio. Chien
Li, et al. Endocrinology Vol. 2003,144(7),
3216-3224 )
showed little or no cross-reactivity. B/B0,
Bound to maximum-bound ratio. Chien
Li, et al. Endocrinology Vol. 2003,144(7),
3216-3224 |
| 
|
| Figure 4. Ucn III-like
ir in secreted media from MIN6 cells. MIN6 cells (106
cells per well) were incubated in media containing
various concentrations of glucose, 30 µM
forskolin, or 30 mM KCl and
supplemented with BSA (0.2% wt/vol) for 4 h. Cultured
supernatants were collected and assayed for Ucn III.
Cells were treated in triplicate, and the average
of duplicate experiments is shown. *, P <
0.05 vs. 5 mM glucose;
**, P < 0.01 vs. 5 mM
glucose. Chien Li, et
al. Endocrinology Vol. 2003,144(7), 3216-3224 |
| 
|

|
| Figure 6. Plasma
glucose (A) and glucagon (B) levels in male rats treated
with vehicle or Ucn III (9 or 90 nmol/kg). Basal glucose
and glucagon concentrations are 5.1 ± 0.2 mmol/liter
and 52.3 ± 3.2 pg/ml, respectively. C, Glucagon levels
at 5 min after vehicle or Ucn III (90 nmol/kg) injection
with or without Ast2-B (90 nmol/kg) pretreatment.
B, *, P < 0.05 vs. vehicle. C, *, P < 0.05 vs.
the rest of groups. Chien Li, et al. Endocrinology
Vol. 2003,144(7), 3216-3224 |
Figure 5. The effects
of either vehicle or Ucn III (0.09–9 nmol/kg,
iv) on plasma glucose (A) and insulin (B) levels in
male rats. C, Insulin levels at 30 min after iv vehicle
or Ucn III (90 nmol/kg) with or without Ast2-B
(90 nmol/kg) pretreatment. Basal glucose level, 4.25
± 0.18 mmol/liter. B, For 9 nmol/kg group: +, P
< 0.05 vs. vehicle; for 90 nmol/kg group:
*, P < 0.05; **, P < 0.01 vs.
vehicle. C, *, P < 0.05 vs. the rest
of groups. Chien Li, et al. Endocrinology Vol. 2003,144(7),
3216-3224 |
| 
|
| Figure 8. Effects of a glucagon
antagonist on Ucn III-induced insulin secretion from
isolated rat islets. Ucn III (1 nM)
and 10 nM glucagon stimulate
insulin secretion. Pretreatment with 1 µM
des-glucagon abolishes the effect of glucagon but
not Ucn III on insulin secretion. Values from islets
incubated in medium with 2.8 mM
glucose serve as controls. *, P < 0.05 compared
with control. +, P < 0.05 vs. control
and glucagon plus des-glucagon. Chien
Li, et al. Endocrinology Vol. 2003,144(7),
3216-3224 |
|
| 
|
| An illustrated scheme of the pathways
involved in Ucn/Ucn II, cAMP induction, and IL-6 output.
Ucns increase intracellular cAMP levels via the stimulation
of adenylate cyclase. To increase IL-6 output, both
PKC and p38 MAPK signaling cascades are involved downstream
of the Ucn/Ucn II-cAMP pathway in A7r5 cells. Kazunori
Kageyama and Toshihiro Suda. Endocrinology
2003, 144(6) 2234-2241 |
| 
|
Effects of CRF family peptides on interleukin-6
levels in A7r5 aortic smooth muscle cells. Cells were
incubated for 48 h with medium alone (control) or
with medium containing CRF (open triangle),
Ucn (open circle), Ucn II (open square),
antisauvagine-30 (closed triangle), 100 nM
of Ucn with increasing concentrations of antisauvagine-30
(closed circle), and 100 nM
of Ucn II with increasing concentrations of antisauvagine-30
(closed square). IL-6 levels in the medium
were measured by ELISA. Results shown are representative
of three independent experiments. Statistical analyses
were performed using one-way ANOVA, followed by Scheffé’s
F post hoc test.
|
%019-30%;%019-29%;%019-27%;%019-28%
|


