|
|
|
Melanocyte Stimulating Hormone |
Hormone Overview

Role of Portal System


R A H Adan et al. The MC4 receptor and control of appetite. British Journal of Pharmacology (2006) 149, 815–827
We found that PRCP is mainly expressed in the lateral hypothalamic Hcrt and MCH neurons. These neurons project to various areas of the hypothalamus, such as the PVN, where α-MSH (1–13) terminals strongly innervate MC4R-expressing neurons. It is our hypothesis that PRCP, once released from the Hcrt and/or MCH terminals, will degrade α-MSH, thus increasing the antagonist effect of agouti-related protein (AgRP) and enhancing the orexigenic tone of the system. In support of this, congenic mice and PRCPgt/gt mice are leaner than the wild-type controls. GHS-R/LR, growth hormone secretagogue receptor/leptin receptor; NPY, neuropeptide Y.
Wallingford et al. J Clin Invest. 2009 Aug 3;119(8):2291-2303.
Product of PREP activity on the substrate α-MSH (1-13) |

|
BACKGROUND: In vitro reactions are useful to identify putative enzyme substrates, but in vivo validation is required to identify actual enzyme substrates that have biological meaning. To investigate in vivo effects of prolyl endopeptidase (PREP), a serine protease, on α melanocyte stimulating hormone (α-MSH), we developed a new mass spectrometry based technique to quantitate, in multiplex, the various forms of α-MSH.
METHODS: Using Multiple Reaction Monitoring (MRM), we analyzed peptide transitions to quantify three different forms of α-MSH. Transitions were first confirmed using standard peptides. Samples were then analyzed by mass spectrometry using a triple quadrupole mass spectrometer, after elution from a reverse phase C18 column by a gradient of acetonitrile.
RESULTS: We first demonstrate in vitro that PREP digests biological active α melanocyte stimulating hormone (α-MSH1-13), by cleaving the terminal amidated valine and releasing a truncated α melanocyte stimulating hormone (α-MSH1-12) product - the 12 residues α-MSH form. We then use the technique in vivo to analyze the MRM transitions of the three different forms of α-MSH: the deacetylated α-MSH1-13, the acetylated α-MSH1-13 and the truncated form α-MSH1-12. For this experiment, we used a mouse model (PREP-GT) in which the serine protease, prolyl endopeptidase, is deficient due to a genetrap insertion. Here we report that the ratio between acetylated α-MSH1-13 and α-MSH1-12 is significantly increased (P-value = 0.015, N = 6) in the pituitaries of PREP-GT mice when compared to wild type littermates. In addition no significant changes were revealed in the relative level of α-MSH1-13 versus the deacetylated α-MSH1-13. These results combined with the demonstration that PREP digests α-MSH1-13 in vitro, strongly suggest that α-MSH1-13 is an in vivo substrate of PREP.
CONCLUSION: The multiplex targeted quantitative peptidomics technique we present in this study will be decidedly useful to monitor several neuropeptide enzymatic reactions in vivo under varying conditions.
Perroud et al. Mol Brain. 2009 Jun 2;2(1):14.
|
|

|
The anorexigenic neuromodulator α-melanocyte-stimulating hormone (α-MSH; referred to here as α-MSH1-13) undergoes extensive posttranslational processing, and its in vivo activity is short lived due to rapid inactivation. The enzymatic control of α-MSH1-13 maturation and inactivation is incompletely understood. Here we have provided insight into α-MSH1-13 inactivation through the generation and analysis of a subcongenic mouse strain with reduced body fat compared with controls. Using positional cloning, we identified a maximum of 6 coding genes, including that encoding prolylcarboxypeptidase (PRCP), in the donor region. Real-time PCR revealed a marked genotype effect on Prcp mRNA expression in brain tissue. Biochemical studies using recombinant PRCP demonstrated that PRCP removes the C-terminal amino acid of α-MSH1-13, producing α-MSH1-12, which is not neuroactive. We found that Prcp was expressed in the hypothalamus in neuronal populations that send efferents to areas where α-MSH1-13 is released from axon terminals. The inhibition of PRCP activity by small molecule protease inhibitors administered peripherally or centrally decreased food intake in both wild-type and obese mice. Furthermore, Prcp-null mice had elevated levels of α-MSH1-13 in the hypothalamus and were leaner and shorter than the wild-type controls on a regular chow diet; they were also resistant to high-fat diet-induced obesity. Our results suggest that PRCP is an important component of melanocortin signaling and weight maintenance via control of active α-MSH1-13 levels.
Wallingford et al. J Clin Invest. 2009 Aug 3;119(8):2291-2303.
|
|

|
MacNeil DJ, et al., Eur J Pharmacol 2002 Apr 12;440(2-3):141-57
|

|
Five G-protein-coupled melanocortin receptors (MC(1)-MC(5)) are expressed
in mammalian tissues. The melanocortin receptors support diverse physiological
functions, including the regulation of hair color, adrenal function, energy
homeostasis, feed efficiency, sebaceous gland lipid production and immune
and sexual function. The melanocortins (adrenocorticotropic hormone (ACTH),
α-melanocyte-stimulating hormone (α-MSH), β-MSH and γ-MSH)
are agonist peptide ligands for the melanocortin receptors and these peptides
are processed from the pre-prohormone proopiomelanocortin (POMC). Peptide
antagonists for the melanocortin MC(1), MC(3) and MC(4) receptors include
agouti-related protein (AgRP) and agouti. Diverse lines of evidence, including
genetic and pharmacological data obtained in rodents and humans, support
a role for the melanocortin MC(3) and MC(4) receptors in the regulation
of energy homeostasis. Recent advances in the development of potent and
selective peptide and non-peptide melanocortin receptor ligands are anticipated
to help unravel the roles for the melanocortin receptors in humans and to
accelerate the clinical use of small molecule melanocortin mimetics.
MacNeil DJ, et al., Eur J Pharmacol 2002 Apr 12;440(2-3):141-57
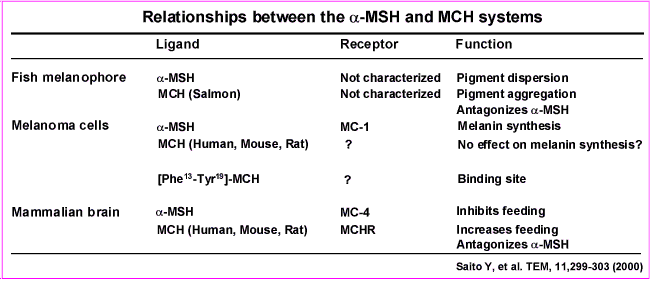
|
|
|
β-MSH
|

|
Harrold JA, et al. Peptides. 2003 Mar;24(3):397-405
|

|
α-Melanocyte stimulating hormone (MSH) has generally been assumed to be the endogenous ligand acting at the melanocortin-4 receptor (MC4-R), activation of which in the hypothalamus leads to reduced feeding. However, β-MSH is also capable of activating MC4-R and inhibiting feeding. Here, we investigated the possibility that β-MSH acts as an endogenous MC4-R agonist and that this melanocortin peptide plays a role in the regulation of feeding and energy balance. We found that β-MSH had significantly higher affinities than α-MSH at both human MC4-R transfected into CHO cells (K(i): β-MSH, 11.40.4 nmol/l versus α-MSH, 32416 nmol/l, P<0.001) and MC4-R in rat hypothalamic homogenates (K(i): β-MSH, 5.00.4 nmol/l versus α-MSH, 22.52.3 nmol/l, P<0.001). Incubation of brain slices with 5 microM β-MSH significantly increased [35S]GTPγS binding by 140-160% (P<0.001), indicating activation of G-protein-coupled receptors (GPCRs), in the hypothalamic ventromedial (VMH), dorsomedial (DMH), arcuate (ARC) and paraventricular (PVN) nuclei. These sites match the distribution of β-MSH immunoreactive fibres and also the distribution of MC4-R binding sites which we and others previously reported. Food-restriction significantly increased β-MSH levels in the VMH, DMH and ARC (all P<0.05) above freely-fed controls, whilst α-MSH concentrations were unchanged. We propose that increased β-MSH concentrations reflect blockade of the peptide's release in these sites, consistent with the increased hunger and the known up-regulation of MC4-R in the same nuclei. Thus, we conclude that (1). β-MSH has higher affinity at MC4-R than α-MSH; (2). β-MSH activates GPCR in these sites, which are rich in MC4-R; and (3). β-MSH is present in hypothalamic nuclei that regulate feeding and its concentrations alter with nutritional state. We suggest that β-MSH rather than α-MSH is the key ligand at the MC4-R populations that regulate feeding, and that inhibition of tonic release of β-MSH is one mechanism contributing to hunger in under-feeding.
Harrold JA, et al. Peptides. 2003 Mar;24(3):397-405
|
|

|
Fricke K., et al. Endocrinology. First published January 13, 2005 as doi:10.1210/en.2004-1097
|

|
We report the isolation of a novel human circulating proopiomelanocortin-derived peptide named VA-ß-MSH from hemofiltrate and its pharmacological characterization. Screening for lipolytic activity in differentiated 3T3-L1 adipocytes led to the isolation from a hemofiltrate peptide library by alternating reversed-phase and cation-exchange chromatography. In the course of this isolation, we also identified human ß-MSH (1-22). We synthesized VA-ß-MSH by the Fmoc (N-(9-fluorenyl)-methoxycarbonyl) solid phase method and used synthetic ß-MSH (1-22) to confirm that both isolated peptides are lipolytically active in a dosedependent manner in differentiated 3T3-L1 adipocytes in the nanomolar range. Using cAMPELISA, we demonstrate that stimulation with both peptides caused a strong cAMP elevation in this cell system. Furthermore, we show that the selective inhibitors of cAMP-dependent protein kinase, Rp-8-CPT-cAMPS and H89, significantly reduce VA-ß-MSH- and ß-MSH (1-22)-mediated lipolysis. Although isolated here following its lipolytic activity on 3T3-L1 cells, this newly identified circulating human melanocortin may also serve other functions in human physiology. Moreover, the fact that these peptides have been identified following a functional assay but have been overseen in large proteomic approaches underscore the importance for such approaches in order to identify previously undescribed circulating bioactive molecules.

Lipolytic potency and intracellular cAMP formation of VA-ß-MSH, ß-MSH (1-22), hACTH (1-39), and forskolin. Results are shown as means (± SD) of three independent experiments.

Lipolytic effect of synthetic VA-ß-MSH and ß-MSH (1-22). Dose-response relationships of increasing concentrations of both peptides. After incubation of 3T3-L1 adipocytes with either peptide for 5 h, glycerol content in the supernatants was measured. Results are expressed as percentages of the lipolytic response of cells to 1 µM isoproterenol and are means ± SD (n=3). One of three independent experiments is presented.
 
Effects of specific PKA inhibitors on VA-ß-MSH- and ß-MSH (1-22)- induced lipolysis. 3T3-L1 adipocytes were preincubated with or without inhibitor for 40 minutes, followed by the incubation with 20 nM VA-ß-MSH or ß-MSH (1-22) for 5 h. A. Preincubation of 3T3-L1 adipocytes with 0.3 mM and 1 mM Rp-8-CPT-cAMPS significantly decreased VA-ß-MSH-induced lipolysis. B. VA-ß-MSH- and ß-MSH (1-22)- induced glycerol release were inhibited in the presence of H89. Basal lipolysis was not significantly changed by either inhibitor. Data points are means ± SD (n=3). Results shown are representative for three separate experiments. * P < 0.05 versus no inhibitor.
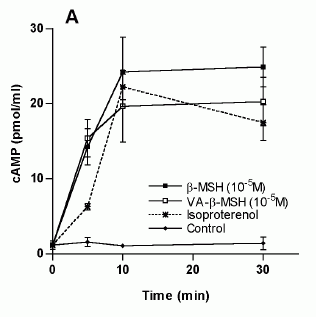 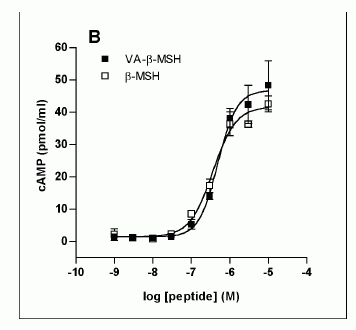
Characterization of the VA-ß-MSH- and ß-MSH (1-22)- stimulated cAMP production in 3T3-L1 adipocytes. The differentiated adipocytes were transferred into fresh DMEM on the day of the experiment, varying concentrations of ß-MSH (1-22) and VA-ß- MSH were added and incubated at 37°C for the indicated time points. The supernatants were 27 removed and cells were lysed with seventy percent ethanol. Cell lysates were assayed for cAMP by ELISA. A. Time dependency of VA-ß-MSH- and ß-MSH (1-22)- stimulated cAMP production in 3T3-L1 cells. Isoproterenol (1 µM) was used as a positive control. B Doseresponse relationship of cAMP-production in differentiated 3T3-L1 cells by VA-ß-MSH andß-MSH (1-22). Each point represents the mean ± SD of triplicate values. Results are representative for three (A) and two (B) independent experiments.

Effect of VA-ß-MSH on CREB and perilipin phosphorylation. A 3T3-L1 adipocytes were serum deprived overnight and subsequently stimulated with 300 nM VA-ß- MSH for the times indicated. At each time point, cells were lysed, and equal amounts of lysate protein were subjected to immunoblot analysis. Blots were probed with antibodies against serine 133-phosphorylated CREB (pCREB) and total CREB. One of three independent experiments each with two replicates is shown. The positions of the pCREB and CREB bands are indicated. B After 15 min stimulation with VA-ß-MSH, cell lysates were subjected to immunoblot analysis, and blots were probed with an antibody against perilipin. The characteristic upward shift observed with VA-ß-MSH reveals the phosphorylation of perilipin A in stimulated cells compared to cells treated with control medium (representative blot of three independent experiments).
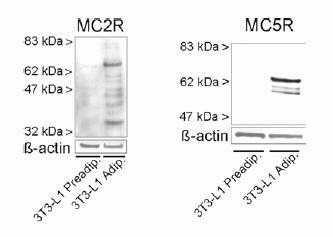
Protein expression of melanocortin receptor isoforms 2 and 5 in 3T3-L1 adipocytes. Untreated 3T3-L1 adipocytes were lysed and subjected to SDS-PAGE followed by immunoblot analysis. Blots were probed using a specific antiserum directed against MC2R (left side) or an affinity-purified antibody against MC5R (right side). ß-actin served as a loading control.
Fricke K., et al. Endocrinology. First published January 13, 2005 as doi:10.1210/en.2004-1097
|
|
|
γ-MSH |

|
Denef C, et al. Ann N Y Acad Sci. 2003 Jun;994:123-32
|

|
The melanocortin (MC) γ3-MSH is believed to signal through the MC3 receptor. We showed that it induces a sustained increase in intracellular free calcium levels ([Ca(2+)](i)) in a subpopulation of pituitary cells. Most of the cells responding to γ3-MSH express more than one pituitary hormone mRNA. The effect of γ3-MSH is blocked by SHU9119, a MC3R and MC4R antagonist, in only 50% of the responsive cells, suggesting that in half of these cells the mediating receptor is not the MC3R. Low picomolar doses of γ3-MSH increase [Ca(2+)](i) in the growth hormone (GH)- and prolactin (PRL)-secreting GH3 cell line. γ2-MSH and α-MSH display a similar effect. SHU9119 does not affect the γ3-MSH-induced [Ca(2+)](i) response. MTII, a potent synthetic agonist of the MC3R, MC4R, and MC5R, also shows no or low potency in increasing [Ca(2+)](i). By means of RT-PCR, the mRNA of the MC2R, MC3R, and MC4R receptors is undetectable. Experiments testing γ2-MSH analogues with single alanine replacements show that, unlike the classic MCRs, the His(5)-Phe(6)-Arg(7)-Trp(8) sequence in γ2-MSH is not a core sequence for activating the γ-MSH receptor in GH3 cells, whereas Met(3) is essential. Low nanomolar doses of γ-MSH increase intracellular cAMP levels. Blockade of protein kinase A abolishes the [Ca(2+)](i) responses to γ3-MSH. γ2-MSH increases binding of [S(35)]GTPγS to membrane preparations of GH3 cells. The pharmacological characteristics of γ-MSH peptides and analogues on [Ca(2+)](i) and the signal-transduction pathways present strong evidence for the expression of a hitherto uncharacterized γ-MSH receptor in GH3 cells, belonging to the G protein-coupled receptor family.
Denef C, et al. Ann N Y Acad Sci. 2003 Jun;994:123-32
|
|

|
Stanley SA, et al. FEBS Lett. 2003 May 22;543(1-3):66-70
|

|
The roles of the melanocortin 3 receptor (MC3-R) and its agonist, γ(2)-melanocyte-stimulating hormone (γ(2)-MSH) in the regulation of the hypothalamo-pituitary-gonadal (HPG) axis are poorly understood. Here we show γ(2)-MSH stimulated intracellular cAMP accumulation and gonadotrophin-releasing hormone (GnRH) secretion in the immortalised GnRH cell line GT(1)-7. The MC3/4-R antagonist Agrp blocked these actions. Reverse transcriptase polymerase chain reaction demonstrated GT(1)-7 cells express MC3-R mRNA. γ(2)-MSH also stimulated GnRH release from hypothalamic explants. In vivo, γ(2)-MSH administration into the medial preoptic area significantly increased plasma luteinising hormone. MC3-R and γ(2)-MSH may modulate the HPG axis.
Stanley SA, et al. FEBS Lett. 2003 May 22;543(1-3):66-70
|
|
|
MSH Related |

|
Oosterom
J, et al. J Biol Chem 276 (2), 931-936, 2001
|

|
[D-Tyr4]-MT
II is an agonist 100 times
selectively binding MC-4R. [Nle4]Lys-γ2-MSH
is an agonist 5 times selectively binding MC-3R.
Oosterom
J, et al. J Biol Chem 276 (2), 931-936, 2001





|
|

|
Haskell-Luevano C, et al. J Med Chem. 2001 Jun 21;44(13):2247-52
|

|
The central melanocortin receptors, melanocortin-4 (MC4R) and melanocortin-3 (MC3R), are involved in the regulation of satiety and energy homeostasis. The MC4R in particular has become a pharmaceutical industry drug target due to its direct involvement in the regulation of food intake and its potential therapeutic application for the treatment of obesity-related diseases. The melanocortin receptors are stimulated by the native ligand, α-melanocyte stimulating hormone (α-MSH). The potent and enzymatically stable analogue NDP-MSH (Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH(2)) is a lead peptide for the identification of melanocortin amino acids important for receptor molecular recognition and stimulation. We have synthesized nine peptide fragments of NDP-MSH, deleting N- and C-terminal amino acids to determine the "minimally active" sequence of NDP-MSH. Additionally, five peptides were synthesized to study stereochemical inversion at the Phe 7 and Trp 9 positions in attempts to increase tetra- and tripeptide potencies. These peptide analogues were pharmacologically characterized at the mouse melanocortin MC1, MC3, MC4, and MC5 receptors. This study has identified the Ac-His-DPhe-Arg-Trp-NH(2) tetrapeptide as possessing 10 nM agonist activity at the brain MC4R. The tripeptide Ac-DPhe-Arg-Trp-NH(2) possessed micromolar agonist activities at the MC1R, MC4R, and MC5R but only slight stimulatory activity was observed at the MC3R (at up to 100 microM concentration). This study has also examined to importance of both N- and C-terminal NDP-MSH amino acids at the different melanocortin receptors, providing information for drug design and identification of putative ligand-receptor interactions.
Haskell-Luevano C, et al. J Med Chem. 2001 Jun 21;44(13):2247-52
|
|

|
Bednarek,
M.A., et al. Biochem. Bioph. Res. Commun. 286, 641-645
(2001)
|

|
A
new cyclic analog of α-MSH is a potent agonist
at human MC-4R with 90-fold selectivity over hMC-3R
and greater than 2000-fold selectivity over hMC-5R.
Bednarek,
M.A., et al. Biochem. Bioph. Res. Commun. 286, 641-645
(2001)
| |
Binding
Assay (IC50, nM) |
Selectivity |
| |
hMC-3R |
hMC-4R |
hMC-5R |
3/4 |
5/4 |
| MC-4R
Agonist |
490±5 |
4.3±.67 |
4600±50 |
114 |
1070 |
| MT
II |
1.6±.09 |
0.07±.02 |
0.89±.01 |
23 |
13 |
|
|
|

Linear Range 0.16-1.83 ng/ml
|
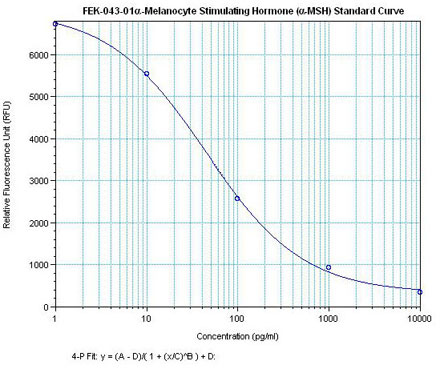
Linear Range 20-384 pg/ml
8 times more sensitive than normal EIA kits
|
|
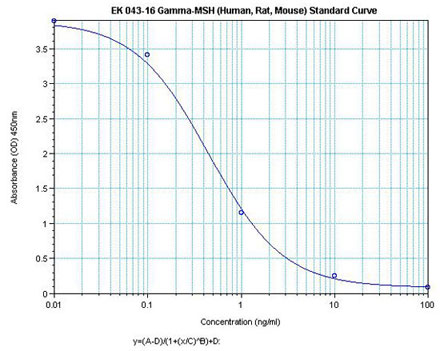
Linear Range 0.1-1.85ng/ml
|

Linear Range 13.7-528 pg/ml
7 times more sensitive than normal EIA kits
|
Olszewski et al. α-Melanocyte stimulating hormone and ghrelin: Central interaction in feeding control
Peptides. 2007 Oct;28(10):2084-9.
Bomberg et al. Functional interaction between nociceptin/orphanin FQ and α-melanocyte-stimulating hormone in the regulation of feeding.
Peptides. 2006 Jul;27(7):1827-34.
Helwig et al. PC1/3 and PC2 gene expression and post-translational endoproteolytic pro-opiomelanocortin processing is regulated by photoperiod in the seasonal Siberian hamster (Phodopus sungorus).
J Neuroendocrinol. 2006 Jun;18(6):413-25.
Kim et al. C-terminal part of AgRP stimulates insulin secretion through calcium release in pancreatic β Rin5mf cells.
Neuropeptides. 2005 Aug;39(4):385-93.
Zou et al. α-melanocyte stimulating hormone protects against H2O2-induced inhibition of wound restitution in IEC-6 cells via a Syk kinase- and NF-κβ-dependent mechanism.
Shock. 2004 Nov;22(5):453-9.
Sasaki et al. Suppression of melanogenesis by induction of endogenous intracellular metallothionein in human melanocytes.
Exp Dermatol. 2004 Aug;13(8):465-71.
Zeh et al. Gain-of-function somatic cell lines for drug discovery applications generated by homologous recombination.
Assay Drug Dev Technol. 2003 Dec;1(6):755-65.
Lassalle et al. Effects of melanogenesis-inducing nitric oxide and histamine on the production of eumelanin and pheomelanin in cultured human melanocytes.
Pigment Cell Res. 2003 Feb;16(1):81-4.
Kim et al. Effects of melanocortin receptor ligands on thyrotropin-releasing hormone release: evidence for the differential roles of melanocortin 3 and 4 receptors.
J Neuroendocrinol. 2002 Apr;14(4):276-82.
Howard et al. Identification of receptors for neuromedin U and its role in feeding.
Nature. 2000 Jul 6;406(6791):70-4.
Kwon et al. Reduced abundance of aquaporins in rats with bilateral ischemia-induced acute renal failure: prevention by α-MSH
Am J Physiol. 1999 Sep;277(3 Pt 2):F413-27.
Chiao et al. α-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats.
J Clin Invest. 1997 Mar 15;99(6):1165-72.
|
|
|
ACTH
%MSH%;%MC4%;%MC-4R%
|
|
|


