|
|
Apelin is an endogenous ligand of the human orphan receptor APJ (orphan G protein-coupled receptor). This peptide is produced through processing from the C-terminal portion in the pre-proprotein consisting of 77 amino acid residues and exists in multiple molecular forms. Although the main physiological functions of apelin have not been clarified yet, it has been demonstrated that apelin partially suppresses cytokine production from mouse spleen and, specifically, induces the promotion of extracellular acidification and inhibition of cAMP production in Chinese hamster ovary cells. Moreover, it is involved in the regulation of blood pressure and blood flow.
De Falco et al. In Vivo 2002 Sep-Oct;16(5):333-6
Apelin gene encodes a pre-proprotein of 77 amino acids, with a signal peptide in the N-terminal region. After translocation into the endoplasmic reticulum and cleavage of the signal peptide, the proprotein of 55 amino acids may generate several active fragments: a 36 amino acid peptide corresponding to the sequence 42-77 (apelin 36), a 31 amino acid peptide corresponding to the sequence 47-77 (apelin 31), a 28 amino acid peptide corresponding to the sequence 50-77 (apelin 28) and a 13 amino acid peptide corresponding to the sequence 65-77 (apelin 13). This latter fragment may also undergo a pyroglutamylation at the level of its N-terminal glutamine residue. However the presence and/or the concentrations of those peptides in human plasma has been questioned. Recently, 46 different apelin peptides ranging from apelin 55 (proapelin) to apelin 12 have been identified in bovine colostrum, including C-ter truncated isoforms.

The orphan receptor APJ and its recently identified
endogenous ligand, apelin, exhibit high levels of mRNA expression in
the heart. However, the functional importance of apelin in the
cardiovascular system is not known. In isolated perfused rat hearts,
infusion of apelin (0.01 to 10 nmol/L) induced a dose-dependent
positive inotropic effect (EC50: 33.1+/-1.5 pmol/L). Moreover,
preload-induced increase in dP/dt(max) was significantly augmented
(P<0.05) in the presence of apelin. Inhibition of phospholipase C
(PLC) with U-73122 and suppression of protein kinase C (PKC) with
stauzrosporine and GF-109203X markedly attenuated the apelin-induced
inotropic effect (P<0.001). In addition, zoniporide, a selective
inhibitor of Na+-H+ exchange (NHE) isoform-1, and KB-R7943, a potent
inhibitor of the reverse mode Na+-Ca2+ exchange (NCX), significantly
suppressed the response to apelin (P<0.001). Perforated
patch-clamp recordings showed that apelin did not modulate L-type
Ca2+ current or voltage-activated K+ currents in isolated adult rat
ventricular myocytes. Apelin mRNA was markedly downregulated in
cultured neonatal rat ventricular myocytes subjected to mechanical
stretch and in vivo in two models of chronic ventricular pressure
overload. The present study provides the first evidence for the
physiological significance of apelin in the heart. Our results show
that apelin is one of the most potent endogenous positive inotropic
substances yet identified and that the inotropic response to apelin
may involve activation of PLC, PKC, and sarcolemmal NHE and
NCX.
Szokodi et al.Circ Res 2002 Sep 6;91(5):434-40
Apelin is
an endogenous ligand of the human orphan receptor APJ. We detected
apelin-like immunoreactivity in the adipocytes, gastric mucosa, and
Kupffer cells in the liver. We also detected apelin-like
immunoreactivity localized within the endothelia of small arteries
in various organs. Further, it was found that mean arterial pressure
after the administration of apelin-12, apelin-13, and apelin-36 at a
dose of 10 nmol/kg in anaesthetized rats was reduced by 26+/-5,
11+/-4, and 5+/-4 mm Hg, respectively. In the presence of a nitric
oxide (NO) synthase inhibitor, the effect of apelin-12 on blood
pressure was abolished. Furthermore, the administration of apelin-12
(10 nmol/kg) in rats produced a transitory elevation of the plasma
nitrite/nitrate concentration from a basal level of 21.4+/-1.6 to
27.0+/-1.5 microM. Thus, apelin may lower blood pressure via a
nitric oxide-dependent mechanism.
Tatemoto et al. Regul Pept 2001 Jun 15;99(2-3):87-92
With the use of an antiserum against human
apelin-36 (Phoenix Pharmaceuticals), apelin-immunoreactivity (irAP)
was detected in neurons and cell processes of the supraoptic nucleus
(SO), paraventricular nucleus (PVH), accessory neurosecretory nuclei
(Acc) and suprachiasmatic nucleus. Strongly labeled cells/processes
were noted in the internal layer of the median eminence,
infundibular stem, anterior and posterior pituitary. Double-labeling
the sections with goat polyclonal neurophysin I-antiserum and rabbit
polyclonal apelin-antiserum revealed a population of magnocellular
neurons in the PVH, SO and Acc expressing both irAP and neurophysin
I-immunoreactivity (irNP), the latter being a marker of
oxytocin-containing neurons. By inference, the AP-positive but
irNP-negative magnocellular neurons could be vasopressin-containing.
The presence of irAP in certain hypothalamic nuclei and pituitary
suggests that the peptide may be a signaling molecule released from
the hypothalamic-hypophysial axis.
Brailoiu et al. Neurosci Lett 2002 Jul
26;327(3):193-7
Apelin is the recently identified endogenous ligand
for the G-protein-coupled receptor, APJ. Preproapelin and APJ mRNA
are found in hypothalamic regions known to be important in the
regulation of food and water intake, and pituitary hormone release.
The effects of intracerebroventricular (ICV) administration of
pyroglutamylated apelin-13 on food and water intake and pituitary
hormone release in rats were investigated. Apelin-13 had little
effect on food intake, but dose-dependently increased drinking
behaviour and water intake at 1 h. Apelin-13 (10 nmol) increased
water intake by up to sixfold compared to saline. Compared to saline
control, apelin-13 (10 nmol) significantly increased plasma ACTH and
corticosterone and decreased plasma prolactin, LH and FSH at 30 min.
In vitro, apelin-13 stimulated the release of CRH and AVP from
hypothalamic explants, but had no effect on NPY release. These
results suggest that apelin may play an important role in the
hypothalamic regulation of water intake and endocrine axes.
Taheri et al. Biochem Biophys Res Commun 2002 Mar 15;291(5):1208-12
Maintenance of human energy homeostasis is
regulated by a complex network. Peptides secreted from the
gastrointestinal tract (GI) are signaling to the brain and other
organs initiating or terminating food intake and energy expenditure.
In the present study we investigated basal plasma levels of apelin,
orexin-A, and leptin in morbid obese patients. In addition, we
measured in a subgroup of these patients in the same individual
orexin-A and leptin plasma levels one year after gastric banding
surgery. METHODS: Basal plasma values were determined in obese
patients (BMI=48+/-1 kg/m2n=32) after an overnight fast and compared
to healthy, normal weighted (BMI=22+/-2 kg/m2n=12) controls. In
addition, blood samples were collected in a subgroup of patients
(BMI=48+/-1 kg/m2n=8) the day before surgery and 1 year after the
operation. Apelin, orexin-A, and leptin levels were analysed using
ELISAs. RESULTS: One year after the operation obese patients
significantly lost weight (from 48+/-2 kg/m2 to 39+/-2 kg/m2;
p<0,001). Apelin, orexin-A and leptin levels in obese patients
were significantly higher compared to control individuals (736+/-50
pg/ml vs. 174+/-14 pg/ml, p<0.0001; 75.3+/-24.1 pg/ml vs.
0.8+/-0.4 pg/ml, p<0.0001; 79.0+/-2.4 ng/ml vs. 5.8+/-0.8 ng/ml,
p<0.0001, respectively). Apelin and leptin plasma concentrations
also correlated significantly with BMI (r=0.769, p<0.0001;
r=0.778; p<0.0001, respectively), while orexin-A correlation was
rather weak (r=0.335, p<0.03). No difference between pre- and
post-operative orexin-A levels was observed, while leptin plasma
levels significantly decreased from 45.1+/-5.4 ng/ml to 27.3+/-6.0
ng/ml (p=0.015). CONCLUSIONS: Apelin, orexin-A, and leptin plasma
levels correlated positively with the BMI. One year after gastric
banding with significant loss in BMI basal plasma levels of leptin
decreased, while orexin-A remained unchanged.

Plasma apelin levels correlated significantly with BMI (r = 0.769; p = 0.0001). Mean value in obese patients (n = 25; 736 ± 50 pg/ml) was significantly higher compared to controls (n = 12; 174 ± 14 pg/ml, p < 0.0001).
Heinonen MV, et al. Regul Pept. 2005 Aug 15;130(1-2):7-13.
Apelin is a recently discovered peptide that is the endogenous ligand for the APJ receptor. The aim of this study
was to characterize apelin expression (mRNA levels) in the rat
gastrointestinal (GI) tract and pancreas, to localize distribution
of apelin peptide containing cells in the stomach by
immunohistochemistry (IHC), to characterize the ontogeny of gastric
apelin expression and peptide, and the influence of apelin on
gastric cell proliferation in vitro. Additionally, the effect of
apelin on cholecystokinin (CCK) secretion, and the involvement of
mitogen-activated protein kinase (MAPK), protein kinase C (PKC) and
changes in [Ca++]i in apelin-induced CCK secretion in
vitro were examined. Northern analysis showed a maximal apelin
expression in the stomach with a lower expression level in the
intestine. Apelin expression was not detected in the pancreas. IHC
revealed abundant apelin positive cells in the glandular epithelium
of the stomach. The ontogeny study showed a higher apelin expression
in the fetal and postnatal rat stomachs when compared to the adult
stomach. In contrast to apelin expression, apelin peptide was not
detected in the rat stomach until 20 days of age and then increased
progressively with age. Apelin was shown to stimulate gastric cell
proliferation in vitro. Apelin also stimulated CCK secretion from a
murine enteroendocrine cell line (STC-1); apelin-stimulated CCK
secretion is mediated through MAPK but not by [Ca++]i
signaling. Together, these data indicate that apelin is an important
new stomach peptide with a potential physiologic role in the GI
tract.
Wang et al. Endocrinology. 2004 Mar;145(3):1342-8.
The results presented herein demonstrate that apelin is
expressed and secreted by both human and mouse adipocytes.
Apelin mRNA levels in isolated adipocytes are close to other
cell types present in white adipose tissue or other organs
known to express apelin such as kidney, heart, and to a lesser
extent brown adipose tissue. Apelin expression is increased
during adipocyte differentiation stage. A comparison of four
different models of obesity in mice showed a large increase in
both apelin expression in fat cells and apelin plasma levels
in all the hyperinsulinemia-associated obesities and clearly
demonstrated that obesity or high fat feeding are not the main
determinants of the rise of apelin expression. The lack of
insulin in streptozotocin-treated mice is associated with a
decreased expression of apelin in adipocytes. Furthermore,
apelin expression in fat cells is strongly inhibited by
fasting and recovered after refeeding, in a similar way to
insulin. A direct regulation of apelin expression by insulin
is observed in both human and mouse adipocytes and clearly
associated with the stimulation of PI3K, PKC and MAPK. These
data provide evidence that insulin exerts a direct control on
apelin gene expression in adipocytes. In obese patients, both
plasma apelin and insulin levels were significantly higher
suggesting that the regulation of apelin by insulin could
influence blood concentrations of apelin. The present work
identifies apelin as a novel adipocyte endocrine secretion and
focuses on its potential link with obesity-associated
variations.
Boucher et al. Endocrinology. 2005 Apr;146(4):1764-71.
The apelin peptide is the endogenous ligand for the apelin G protein-coupled receptor. The distribution of the apelin peptides and receptor are widespread in the central nervous system and periphery, with reported roles in the hypothalamic-pituitary-adrenal axis, blood pressure regulation and as one of the most potent positive inotropic substances yet identified. In this report, we show that in native tissues preproapelin exists as a dimer. Dimeric preproapelin was reduced to monomers by dithiothreitol treatment, indicating disulfide linkages. To evaluate the role of the carboxyl-terminal phenylalanine in the hypotensive action of apelin-13, analogs were generated and tested for their role on blood pressure regulation. Injections of apelin-13 and apelin-12 (15 microg/kg) into spontaneously hypertensive rats lowered systolic and diastolic blood pressure to result in decreases of approximately 60% and 15% in mean arterial blood pressure, respectively. Apelin-13(13[D-Phe]) treatment did not differ from apelin-13 in either efficacy or duration of effect, whereas apelin-13(F13A) revealed a loss of function. However, concomitant administration of apelin-13(F13A) (30 microg/kg) blocked hypotensive effects of apelin-13 (15 microg/kg), which revealed that apelin-13(F13A) behaved as an apelin-specific antagonist.
Lee et al. Endocrinology. 2005 Jan;146(1):231-6.
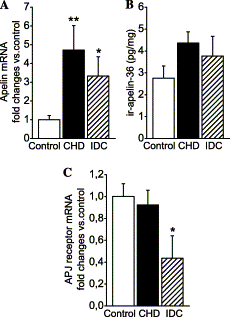
The orphan receptor APJ and its recently identified endogenous ligand, apelin, are expressed in the heart. However, their
importance in the human cardiovascular system is not known. This study shows that apelin-like immunoreactivity is abundantly present in healthy
human heart and plasma. Gel filtration HPLC analysis revealed that atrial and plasma levels of high molecular weight apelin, possibly proapelin,
were markedly higher than those of mature apelin-36 itself. As assessed by quantitative RT-PCR analysis, left ventricular apelin mRNA levels were
increased 4.7-fold in chronic heart failure (CHF) due to coronary heart disease (p<0.01) and 3.3-fold due to idiopathic dilated cardiomyopathy
(p<0.05), whereas atrial apelin mRNA levels were unchanged. Atrial and plasma apelin-like immunoreactivity (using Phoenix's Apelin-36 (Human)
RIA Kit) as well as atrial and ventricular APJ receptor mRNA levels were significantly decreased in CHF. Our results suggest that a new
cardiac regulatory peptide, apelin, and APJ receptor may contribute to the pathophysiology of human CHF.
Atrial and ventricular immunoreactive apelin levels
Apelin-like immunoreactivity was detected in the
heart of organ donors, being over 200-fold higher in the right atria
than in the left ventricles (650 ± 145 pg/mg, n=5 and 2.8 ± 0.6 pg/mg, n=10,
respectively). There was a tendency for left ventricular ir-apelin
levels to be higher in patients with heart failure due to coronary
heart disease (4.4 ± 0.5 pg/mg, p=0.07) and
idiopathic dilated cardiomyopathy (3.8 ± 0.9 pg/mg, p=0.4) than those in controls, but these changes were not
statistically significant (Fig. 1B). Interestingly, atrial ir-apelin
levels were significantly decreased in patients with heart failure
(Fig. 2B).
Plasma apelin levels in normal subjects and in patients with heart failure
Immunoreactive apelin was found to be present in normal human plasma (mean, 89.8 ± 5.3 pg/ml, n=6). Plasma levels of
ir-apelin were significantly decreased in patients with heart failure due to coronary heart disease compared to normal subjects
(in NYHA III: 71 ± 6 pg/ml, p<0.05). Plasma ir-apelin levels showed significant correlation to atrial ir-apelin levels (R=0.4, n=38, p<0.05).
|
|

|
Left ventricular apelin mRNA
(A), immunoreactive apelin-36
(B), and APJ receptor mRNA
(C) levels in
control subjects (n=9) and patients with end-stage heart
failure due to coronary heart disease (CHD, n=7) and
idiopathic dilated cardiomyopathy (IDC, n=6). The mRNA
results are expressed as ratios to 18S RNA determined by TaqMan
real-time quantitative RT-PCR analysis. Bars indicate
means ?nbsp;SEM. *p<0.05 and **p<0.01 vs.
control subjects.
|
Right atrial apelin mRNA (A), immunoreactive apelin-36 (B), and APJ receptor mRNA (C) levels in control subjects (n=6) and patients with heart
failure (NYHA functional class II or III) due to coronary heart disease (CHD, n=38). Bars indicate means ?nbsp;SEM. *p<0.05 and **p<0.01 vs. control subjects.
|
|

|
| Gel filtration HPLC analysis of immunoreactive
apelin in plasma (A,B) and atrial extracts (C,D) of a healthy
control (A,C) and a patient with heart failure (B,D). The arrows
denote elution positions of bovine serum albumin
(V0), apelin-36, and 125I-,
used for the calibration of the column. |
IDC, idiopathic dilated cardiomyopathy; CCHD, coronary heart disease.
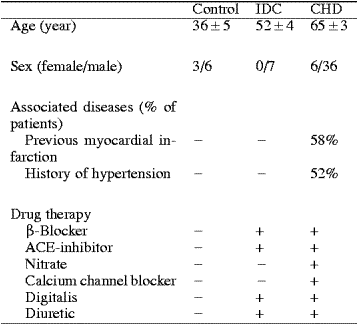
Foldes G, et al. Biochem Biophys Res Commun. 2003 Aug 29;308(3):480-5
Apelin is an endogenous ligand of the human orphan receptor APJ (orphan G protein-coupled receptor). This peptide is produced
through processing from the C-terminal portion in the pre-proprotein consisting of 77 amino acid residues and exists in multiple molecular
forms. Although the main physiological functions of apelin have not been clarified yet, it has been demonstrated that apelin partially suppresses
cytokine production from mouse spleen and, specifically, induces the promotion of extracellular acidification and inhibition of cAMP production
in Chinese hamster ovary cells. Moreover, it is involved in the regulation of blood pressure and blood flow. In this study we have analyzed, by
immunohistochemistry, apelin distribution in several human tissues, demonstrating that apelin has a widespread pattern of expression. These
results seem to confirm that apelin functions widely in various tissues interacting with its specific receptor APJ.
Tissue and blood sampling. After
removal, cardiac tissue samples were blotted dry, immersed in liquid
nitrogen, and stored at -80 °C until assayed. For plasma
sampling, blood was taken before surgery after 30 min bed rest
and collected into chilled tubes containing EDTA. The plasma was
separated by centrifugation at +4 oC and kept at -80 oC
until assayed.
Radioimmunoassay for apelin. Immunoreactive apelin
(ir-apelin) was determined from extracted plasma and right atrial
and left ventricular samples using an apelin-36 radioimmunoassay kit
(Phoenix, Cat-No. RK-057-15). Apelin assay was performed according
to the manufacturer's instructions. The sensitivity of assay was
1 fmol/tube. Tissue peptide levels are expressed as a
concentration per mg wet weight.
HPLC analysis. Gel filtration high performance
liquid chromatography (GF-HPLC) and apelin radioimmunoassay were
used in order to characterize the molecular form of immunoreactive
apelin in plasma and tissue extracts. Aliquots of the atrial tissue
homogenates, used for RNA extraction, from healthy subjects and
patients with heart failure were diluted to 400 ul of
40% acetonitrile in aqueous 0.1% TFA. Plasma samples (1 ml)
were extracted with SepPak C18 cartridges, dried, and reconstituted
in 400 ul of 40% acetonitrile in
aqueous 0.1% TFA. The samples were passed through Millex HV filters
(Millipore) before being loaded into the 7.8 ?nbsp;300 mm
ProteinPak-125 column (Waters). The column was eluted with 40%
acetonitrile in aqueous 0.1% TFA at 1 ml/min. Fractions of
0.5 ml were collected, dried in Savant SpeedVac, and subjected
in duplicate to apelin radioimmunoassay. The column was calibrated
with bovine serum albumin (void volume), apelin-36, and
125I- (total volume).
De Falco M, et al. In Vivo 2002 Sep-Oct;16(5):333-6
The apelin peptide was recently discovered and demonstrated to be the endogenous ligand for the G protein-coupled receptor, APJ. A search of the GenBank databases retrieved a rat expressed sequence tag partially encoding the preproapelin sequence. The GenBank search also revealed a human sequence on chromosome Xq25-26.1, containing the gene encoding preproapelin. We have used the rat sequence to screen a rat brain cDNA library to obtain a cDNA encoding the full-length open reading frame of rat preproapelin. This cDNA encoded a protein of 77 amino acids, sharing an identity of 82% with human preproapelin. Northern and in situ hybridization analyses revealed both human and rat apelin and APJ to be expressed in the brain and periphery. Both sequence and mRNA expression distribution analyses revealed similarities between apelin and angiotensin II, suggesting they that share related physiological roles. A synthetic apelin peptide was injected intravenously into male Wistar rats, resulting in immediate lowering of both systolic and diastolic blood pressure, which persisted for several minutes. Intraperitoneal apelin injections induced an increase in drinking behavior within the first 30 min after injection, with a return to baseline within 1 h.
Lee et al. J Neurochem. 2000 Jan;74(1):34-41.
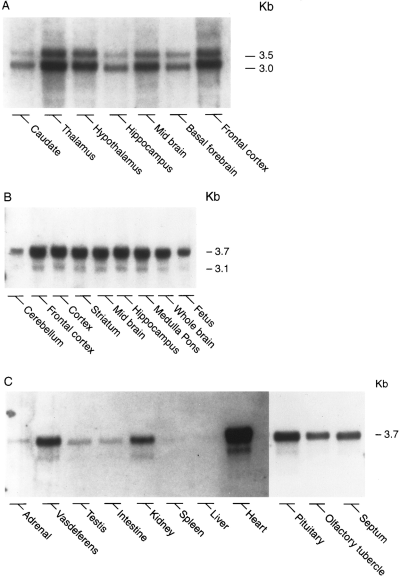
Northern blot analysis of the distribution of preproapelin mRNA in human (A) and rat (B and C) tissues. Each lane contained 10 ug of poly(A)+ RNA
isolated from various tissues, with the exception of rat pituitary (7.5 ug), olfactory tubercle (7.8 ug), septum (5 ug), and liver (7 ug).
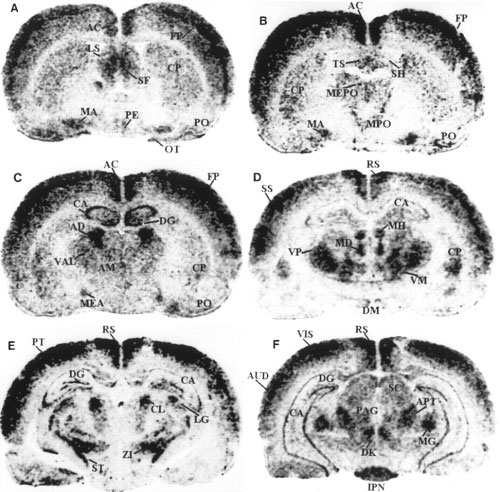
Darkfield autoradiograms of coronal sections
of rat brain showing the localization of preproapelin mRNA, at
various distances from bregma according to the stereotactic
coordinates of Paxinos and Watson (1982).
Shown are representative sections at levels relative to the bregma
at -0.3 mm (A), -0.5 mm (B), -1.8 mm (C), -2.8
mm (D), -3.9 to -4.2 mm (E), and -5.8 mm (F).
3v, third ventricle; 4v, fourth ventricle; 7, facial nucleus; AC,
anterior cingulate cortex; AD, anterodorsal thalamic nucleus; AM,
anteromedial thalamic nucleus; APT, anterior pretectal area; AUD,
auditory area; CA, field of Ammon's horn; Ch, choroid plexus; CL,
centrolateral thalamic nucleus; CP, caudate putamen; DG, dentate
gyrus; DK, nucleus of Darkschewitsch; DM, dorsomedial hypothalamic
nucleus; FP, frontoparietal cortex; IC, inferior colliculus; IPN,
interpeduncular nucleus; LG, lateral geniculate complex; LS, lateral
septal nucleus; MA, magnocellular preoptic nucleus; MD, mediodorsal
thalamic nucleus; MEA, medial amygdaloid nucleus; MEPO, median
preoptic nucleus; MG, medial geniculate nucleus; MH, medial
habenular nucleus; MPO, medial preoptic area; OB, olfactory bulb;
OT, olfactory tubercle; PAG, periaqueductal gray; PB, parabrachial
nucleus; PE, periventricular hypothalamic nucleus; Pi, pineal gland;
PO, primary olfactory cortex; PT, parietal region; PVN,
paraventricular hypothalamic nucleus; RS, retrosplenial area; S5,
sensory root of the trigeminal nerve; SC, superior colliculus; SF,
septofimbrial nucleus; SH, septohypothalamic nucleus; SO, supraoptic
hypothalamic nucleus; SS, primary somatosensory area; ST/STH,
subthalamic nucleus; SUB, dorsal subiculum; TS, triangular septal
nucleus; V, vestibular nucleus; VAL, ventral anterior-lateral
complex thalamus; VIS, primary visual area; VM, ventromedial
thalamic nucleus; VP, ventroposterior thalamic nucleus; ZI, zona
incerta.
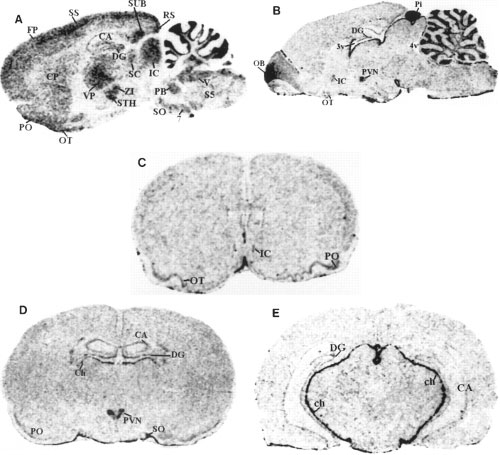
Darkfield autoradiograms of sagittal and coronal sections of rat brain showing the localization of preproapelin mRNA (A) and APJ receptor
mRNA (B-E). A and B show lateral representative sections at 1.77 and 0.4 mm, respectively. Also shown are representative sections at levels relative to the bregma at 1.2 mm (C), -1.8 mm
(D), and -4.8 mm (E). See Fig. 3 for abbreviation definitions.

Blood pressure and heart rate changes after 1 and 2 g/300 g B.W. intravenous apelin injection into rats (n = 5). Values are shown as means ?SEM.
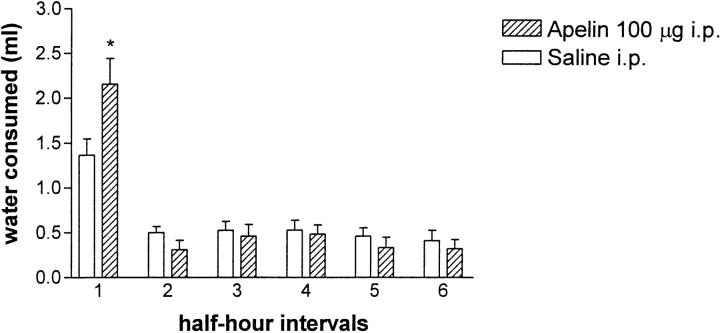
Water consumption after intraperitoneal injection of 100 ug of apelin into rats (n = 18), recorded at 30-min intervals. An asterisk indicates a significant difference in water consumption between vehicle- and apelin-injected rats. Values are shown as means ?SEM.
Lee, Dennis K., et al. Characterization of Apelin, the Ligand for the APJ Receptor. Journal of Neurochemistry 74 (1), 34-41.
In the search for an endogenous ligand of the human orphan receptor APJ, we have isolated from bovine stomach extracts a novel 36-amino acid peptide, designated apelin. The APJ receptor is one of the G protein-coupled orphan receptors, many of which have been considered to be specific receptors for unidentified hormones and neuropeptides. We recently found the presence of apelin in the adipocytes and vascular walls as well as in the stomach. We examined biological activities of apelin and found that apelin lowered blood pressure in rats and also released cholecystokinin(CCK) from dispersed intestinal endocrine cells. Since apelin is an endogenous ligand for the HIV entry coreceptor APJ, we tested the effect of apelin on the entry of HIV in association with CD4, and found that apelin blocked the entry of HIV-1 and HIV-2.
Takemoto et al. Nippon Rinsho. 2000 Mar;58(3):737-46.
We have recently identified apelin as the endogenous ligand for human APJ. In rats, the highest expression of APJ mRNA was detected in the lung, suggesting that APJ and its ligand play an important role in the pulmonary system. When apelin-36 and its pyroglutamylated C-terminal peptide, [<Glu(65)]apelin-13, were compared in microphysiometric analyses, the elevation of extracellular acidification induced in cells expressing APJ by [<Glu(65)]apelin-13 was transient, whereas that by apelin-36 was sustained. These responses were almost completely inhibited by a specific inhibitor for G(i) or that for Na(+)/H(+) exchanger. (125)I -Labeled [<Glu(65)]apelin-13 analogue specifically bound to APJ with a high affinity, and [<Glu(65)]apelin-13 was more potent than apelin-36 in competitive inhibition assays. Because pretreatment with apelin-36 but not [<Glu(65)]apelin-13 drastically reduced the binding of the labeled apelin to APJ, the different patterns of acidification induced by these two peptides appeared to reflect their dissociation rather than association with APJ. Apelin elicited the migration of APJ-expressing cells, and [<Glu(65)]apelin-13 was more potent than apelin-36 in this activity. Heterogeneous molecular forms of apelin corresponding to apelin-36 and [<Glu(65)]apelin-13 were produced in bovine colostrum. Apelin-36 and [<Glu(65)]apelin-13 might have different functions in vivo and in vitro.
Hosoya et al. J Biol Chem. 2000 Jul 14;275(28):21061-7.
The orphan G protein-coupled receptor APJ has been shown to be a coreceptor for human and simian immunodeficiency virus (HIV and SIV) strains. We have determined that some HIV and SIV strains use APJ as a coreceptor to infect the brain-derived NP-2/CD4 cells. Because apelin is an endogenous ligand for the APJ receptor, we examined the inhibitory effects of apelin peptides on HIV infection, and found that the apelin peptides inhibit the entry of some HIV-1 and HIV-2 into the NP-2/CD4 cells expressing APJ. The inhibitory efficiency has been found to be in the order of apelin-36>apelin-17>apelin-13>apelin-12.
Zou et al. FEBS Lett 2000 May 4;473(1):15-8.
The apelin peptide was recently discovered and demonstrated to be the endogenous ligand for the G protein-coupled receptor, APJ. A search of the GenBank databases retrieved a rat expressed sequence tag partially encoding the preproapelin sequence. The GenBank search also revealed a human sequence on chromosome Xq25-26.1, containing the gene encoding preproapelin. We have used the rat sequence to screen a rat brain cDNA library to obtain a cDNA encoding the full-length open reading frame of rat preproapelin. This cDNA encoded a protein of 77 amino acids, sharing an identity of 82% with human preproapelin. Northern and in situ hybridization analyses revealed both human and rat apelin and APJ to be expressed in the brain and periphery. Both sequence and mRNA expression distribution analyses revealed similarities between apelin and angiotensin II, suggesting they that share related physiological roles. A synthetic apelin peptide was injected intravenously into male Wistar rats, resulting in immediate lowering of both systolic and diastolic blood pressure, which persisted for several minutes. Intraperitoneal apelin injections induced an increase in drinking behavior within the first 30 min after injection, with a return to baseline within 1 h.
Lee et al. J Neurochem. 2000 Jan;74(1):34-41.
By using a strategy that we have developed to search for the ligands of orphan seven-transmembrane-domain receptors [S. Hinuma et al., Nature 393 (1998) 272-276], we have recently identified a natural ligand, apelin, for the orphan 7TMR, APJ [K. Tatemoto et al., Biochem. Biophys. Res. Commun. 251 (1998) 471-476]. In this paper, we isolated rat and mouse apelin cDNAs, and analyzed the tissue distribution of apelin mRNA in rats. Although apelin mRNA was widely detected in a variety of tissues, the highest expression of apelin mRNA was detected in the mammary gland of pregnant rats. In the mammary gland, biologically active apelin and its mRNA considerably increased during pregnancy and lactation, and reached a maximal level around parturition. Moreover, a large amount of apelin (14-93 pmol/ml) was found to be secreted in the bovine colostrum, and it was still detectable even in commercial bovine milk. Since apelin partially suppressed cytokine production by mouse spleen cells in response to T cell receptor/CD3 cross-linking, the oral intake of apelin in the colostrum and milk might modulate immune responses in neonates.
Habata et al. Biochim Biophys Acta. 1999 Oct 13;1452(1):25-35.
1. We have determined the binding characteristics of [(125)I]-(Pyr(1))Apelin-13, a putative ligand for the APJ orphan receptor in human cardiovascular and rat tissue and investigated the functional properties of (Pyr(1))Apelin-13 in human saphenous vein. 2. The binding of [(125)I]-(Pyr(1))Apelin-13 to sections of human heart tissue was time dependent and rapid at 23 degrees C. Data were fitted to a single site model with an association rate constant (k(obs)) of 0.115 min(-1). [(125)I]-(Pyr(1))Apelin-13 also dissociated from a single site with a dissociation rate constant of 0.0105 min(-1). 3. In saturation binding experiments [(125)I]-(Pyr(1))Apelin-13 bound to human left ventricle with a K(D) value of 0.35+/-0.08 nM, B(max) of 4.3+/-0.9 fmol mg(-1) protein with a Hill slope of 0.97+/-0.04 and to the right atria with a K(D) of 0.33+/-0.09 nM, B(max) of 3.1+/-0.6 fmol mg(-1) protein and a Hill slope of 0.93+/-0.05. 4. [(125)I]-(Pyr(1))Apelin-13 binding sites were localized using autoradiography to human cardiovascular tissue, including coronary artery, aorta and saphenous vein grafts. In rat tissue a high density of receptors were localized to the molecular layer of the rat cerebellum, rat lung, rat heart and low levels in the rat kidney cortex. 2. (Pyr(1))Apelin-13 potently contracted human saphenous vein with a pD(2) value of 8.4+/-0.2 (n=8). The maximum response elicited by the peptide was 22.6+/-6% of 100 mM KCl. 6. We provide the first evidence of APJ receptor expression, relative densities and functional properties of (Pyr(1))Apelin-13 in human cardiovascular tissue.
Katugampola et al. Br J Pharmacol. 2001 Mar;132(6):1255-60.
Dr. Nae J. Dun,
East Tennessee State
University |
|

|
| Tissue Sample |
Rat
Hypothalamus |
| Fixative |
4%
paraformaldehyde/ 0.2% picric acid in PBS |
| Embedding |
paraffin |
| Control |
No
primary antibody and pre-absorption of the antibody with
the peptide apelin-36 (1µg/ml) |
| Blocking |
2%
Normal Goat Serum |
| Primary Antibody |
Anti-Apelin-36 (Human) Antibody (Catalog
No.: H-057-15) |
| Optimal Dilution |
1:3000, overnight at 4ºC |
| Secondary Antibody |
Goat
Anti-Rabbit IgG, Biotinylated (1:50), 30 min |
| Amplification |
Streptavidin-HRP (Vector), 1:400, 30
min |
| Detection System |
HRP |
| Substrate |
DAB
(Sigma), 3 min |
|
Rat hypothalamus was stained by
Anti-Apelin-36
(Human) Serum (H-057-15) |
|
  |
|
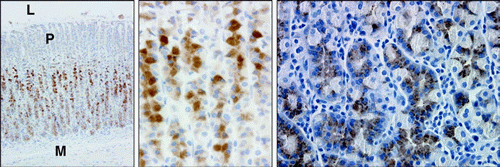
Left. IHC localization of apelin
containing cells in the oxyntic mucosa of the adult rat stomach.
Strong apelin staining (red-brown) is observed in the deep glandular
epithelium of the fundus.
Middle. A high-power
magnification of stomach apelin containing cells.
Right. IHC localization of apelin containing cells in
the adult human stomach. This tissue was devoid of any
pathophysiology. Apelin staining (brown) is observed in the
glandular epithelium. No staining was observed with use of
pre-immune serum (not shown). Micrographs were captured with a Nikon
Eclipse E600 microscope with Plan Fluor, X100, X200, X200,
respectively. L= stomach lumen, P= region of gastric pits, M= muscle
layer of stomach.
Wang et al. Endocrinology. 2004 Mar;145(3):1342-8.
|
|

|
High Power micrograph showing apelin
storage vesicles in a gastric gland of rat stomach. Apelin-staining
vesicles are packed adjacent to the lumen (L) of the gastric gland
suggesting release of apelin into the stomach lumen,
X400.
|
Wang et al. Endocrinology. 2004 Mar;145(3):1342-8.
|
|

|
Ontogeny of apelin peptide in the
oxyntic mucosa of the developing rat stomach. Apelin containing
cells (brown color) were observed initially in the rat stomach at 20
days of age by IHC. IHC failed to detect apelin peptide in stomach
specimens harvested from pups at 2, 7 and 13 days of age (data not
shown). The number of apelin containing cells increased
progressively with age.
Photos were captured with a 10X
objective, M = muscle layer of stomach; E = gastric mucosal
epithelium
Oxyntic mucosa of the developing rat stomach was
stained using Phoenix's anti-Apelin-36 (Human) antiserum (Catalog
Number: H-057-15).
|
Wang et al. Endocrinology. 2004 Mar;145(3):1342-8.
|

Top: Ontogeny of apelin expression (mRNA levels) in the developing rat stomach. Northern hybridization shows the profile of apelin expression in the fetal and postnatal rat stomachs compared with the adult rat stomach. Notice that apelin expression is barely discernible in the adult stomach on this film exposure.
Bottom: Real-time RT-PCR analysis of apelin expression in the fetal, postnatal, and adult rat stomach.
Wang et al. Endocrinology. 2004 Mar;145(3):1342-8.
|
 |
Apelin-like immunoreactivity (Apelin-12 (Human, Mouse, Rat, Bovine) Antiserum, Catalog No.: H-057-23, Phoenix Pharmaceuticals) vascular endothelial cells of large conduit vessels. Photomicrographs show staining in saphenous vein (a) and coronary artery (c) with higher magnification of the respective vessels in (b) and (d).
Kleinz et al. Regul Pept. 2004 May 15;118(3):119-25. |
 |
 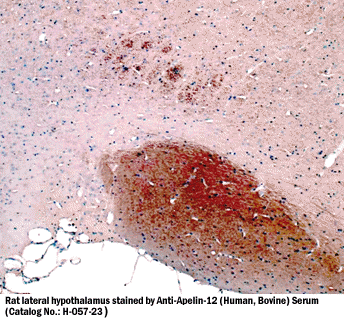
|
Apelin-12-ir in rat hypothalamus |
| Tissue Sample |
Rat lateral
hypothalamus |
| Fixative |
10%
Formalin |
| Embedding |
Paraffin |
| Negative
control |
No primary
antibody |
| Pretreatment |
Target Retrieval
25 min (Steam) |
| Blocking |
2% Normal Goat
Serum |
| Primary
Antibody |
Rabbit
Anti-Apelin-12 (Human, Bovine) Antiserum (Catalog
No.:H-057-23) |
| Optimal
Dilution |
1:3000 (1hour at
RT) |
| Secondary
Antibody |
Goat anti-Rabbit
IgG, Biotinylated (1:400) |
| Amplification |
Streptavidin-HRP (Vector),
1:400, 30 min |
| Detection
system |
HRP |
| Substrate |
DAB
(Sigma) |
| Counterstained |
Hematoxylin |
|
| Apelin-12-ir in rat
medial forebrain boundle |
| Tissue Sample |
Rat medial
forebrain boundle |
| Fixative |
10%
Formalin |
| Embedding |
Paraffin |
| Negative
control |
No primary
antibody |
| Pretreatment |
Target Retrieval
25 min (Steam) |
| Blocking |
2% Normal Goat
Serum |
| Primary
Antibody |
Rabbit
Anti-Apelin-12 (Human, Bovine) Antiserum (Catalog
No.:H-057-23) |
| Optimal
Dilution |
1:3000 (1hour at
RT) |
| Secondary
Antibody |
Goat anti-Rabbit
IgG, Biotinylated (1:400) |
| Amplification |
Streptavidin-HRP (Vector),
1:400, 30 min |
| Detection
system |
HRP |
| Substrate |
DAB
(Sigma) |
| Counterstained |
Hematoxylin |
|
|

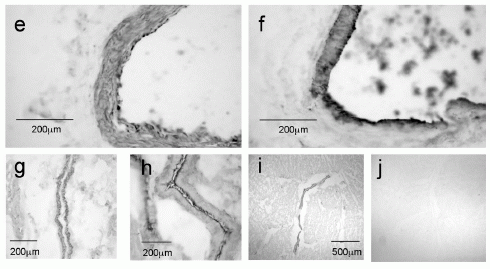
Photomicrographs demonstrating apelin-like immunoreactivity (apelin-LI) in the human heart. Apelin-LI is expressed in vascular endothelial cells of right atria (a and b) and left ventricle (c and e), with adjacent sections (d and f) stained for the endothelial marker von Willebrand factor (vWF). Apelin-LI was also identified in endocardial endothelial cells lining the right atrium (g) with an adjacent section stained for vWF (h). In sections from right atrium (i) no staining was detectable when the primary antiserum was omitted as negative control (j).
Kleinz et al. Regul Pept. 2004 May 15;118(3):119-25.
|

Photomicrographs showing apelin-like immunoreactivity in vascular endothelial cells of blood vessels from kidney (a, b) and adrenal gland (c) with an adjacent section of adrenal gland (d), where the primary antiserum was omitted as negative control.
Kleinz et al. Regul Pept. 2004 May 15;118(3):119-25. |
| |

Apelin-like immunoreactivity in vascular endothelial cells of large conduit vessels. Photomicrographs show staining in saphenous vein (a) and coronary artery (c) with higher magnification of the respective vessels in (b) and (d).
Kleinz et al. Regul Pept. 2004 May 15;118(3):119-25.
|

Figure 1. Apelin-36 Antibody Immunoblot Analyses. Immunoblot analysis of whole cell lysates using the apelin-36 antibody (from Phoenix Pharmaceuticals). (A) Shown are samples from COS-7 cells expressing rat preproapelin (lane 1) and heart tissue samples for a control mouse (lane 2) and a preproapelin-transgenic littermate (lane 3). (B) Samples from COS-7 cells expressing rat preproapelin either untreated (lane 1) or treated with 50 mM DTT and 100 mM IAA for 30 min at 60 °C (lane 2). For all lanes, 25 µg of protein was used. The immunoblots shown are representative of two independent experiments.
Lee et al. Endocrinology. 2005 Jan;146(1):231-6.
|
Only 5 to 40 µL of sample are necessary for accurate peptide level determination using Phoenix’s Magnetic Bead RIA kits. Please contact us at support@phoenixpeptide.com for more information on this product.
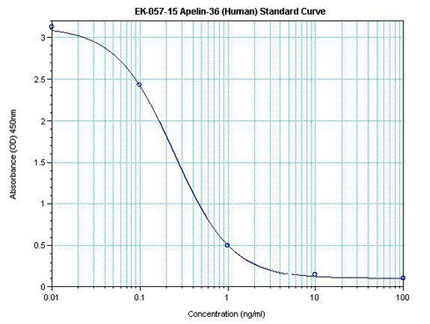 |  | Linear Range: 0.08-1.48 ng/ml
Extraction Free | Linear Range: 20-480 pg/ml
4 times more sensitive than normal EIA kits |
 | 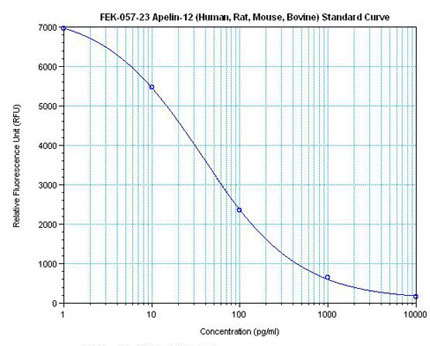 |
Linear Range: 0.07-0.79 ng/ml
| Linear Range: 15.8-419 pg/ml
4 times more sensitive than normal EIA kits |
 |  |  |
Linear Range: 0.26-2.0 ng/ml
Extraction Free
| Linear Range: 5.7 - 346 pg/ml
45 times more sensitive than normal EIA kits | Linear Range: 12.7 - 126 pg/ml
20 times more sensitive than normal EIA kits |

Amino acid sequences of apelin-13 (a) and apelin-36 (b). Dark grey indicates amino acids identical in both peptides. The N-terminal Pyr1 of (Pyr1) apelin-13 differs from the prepropeptide sequence as a result of post-translational modification. The antiserum used for immunocytochemistry was raised against the C-terminal dodecapeptide common to both peptides, which is indicated in dark grey.
Kleinz et al. Regul Pept. 2004 May 15;118(3):119-25.
|

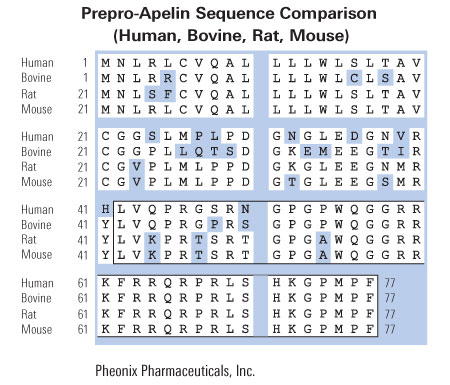


A: An alignment of amino acid sequences of rat preproapelin with human and bovine preproapelin. Conserved amino acids are shown boxed. The mature apelin peptide is shaded. Numeric amino acid positions are indicated on the right. The GenBank accession numbers for rat and human preproapelin are AF179679 and AF179680, respectively.
B: An alignment of amino acid sequences of human apelin and angiotensin II. Conserved amino acids are shown boxed. C: The genomic structure of the human preproapelin gene as found in the human PAC 454M7 clone (GenBank accession no. AL022162). Nucleotide positions of PAC 454M7 defining preproapelin gene exons (boxes) are shown at the top. The ORF is shown in black, with the nucleotide positions of the start and stop codons shown at bottom.
Lee et al. J Neurochem. 2000 Jan;74(1):34-41.
Tips: See More Research Abstracts, Antibody Stainings, Immunoassay Kits Curves and Sequences by clicking the tabs on the top.
|
|
|
APJ_Receptor;SCNH2
%apelin%
|
|
|


