|
|
|
Galanin and Its Antagonists |
Galanin is a neuropeptide encoded by the GAL gene, that is widely expressed in the brain, spinal cord, and gut of humans as well as other mammals. Galanin signaling occurs through three G protein-coupled receptors.
The functional role of galanin remains largely unknown; however, galanin is predominately involved in the modulation and inhibition of action potentials in neurons. Galanin has been implicated in many biologically diverse functions, including: nociception, waking and sleep regulation, cognition, feeding, regulation of mood, regulation of blood pressure, it also has roles in development as well as acting as a trophic factor. Galanin is linked to a number of diseases including Alzheimer’s disease, epilepsy as well as depression, eating disorders and cancer. Galanin appears to have neuroprotective activity as its biosynthesis is increased 2-10 fold upon axotomy in the peripheral nervous system as well as when seizure activity occurs in the brain. It may also promote neurogenesis.
Galanin is predominantly an inhibitory, hyperpolarizing neuropeptide and as such inhibits neurotransmitter release. Galanin is often co-localized with classical neurotransmitters such as acetylcholine, serotonin, and norepinephrine, and also with other neuromodulators such as Neuropeptide Y, Substance P, and Vasoactive intestinal peptide.
|

|
|

|
|
|
|
Peptide Name
|
Function
|
Tracer
|
Tissue or
cell
|
|
References
|
|
galantide (M15), galanin-(1-12) -Pro-SP-(5-11) amide
|
antagonist
|
|
rat hypothalamus
|
KD(1) less than 0.1 nM and KD(2) approximately 6 nM
|
Langel U, et al. Int J Pept Protein Res. 1992 ; 39(6)
:516-22
|
|
galanin
|
agonist
|
[125I] Galanin
|
rat pancreatic beta-cell line Rin m 5F
|
KD: 1 nM
|
Kask K, et al. Regul Pept. 1995 , 59(3):341-8
|
|
galanin-(1-16)
|
agonist
|
[125I] galanin
|
rat forebrain and spinal cord
|
IC50: 3 nM
|
Fisone G, et al. Proc Natl Acad Sci U S A. 1989; 86(23)
:9588-91
|
|
M35 [galanin(1-13)- bradykinin (2-9) amide]
|
antagonist
agonist ? |
[125I] M35
|
rat pancreatic beta-cell line Rin m 5F
|
KD = 0.9 +/- 0.1 nM; Bmax=72 +/- 3 fmol/mg protein
|
Kask K, et al. Regul Pept. 1995, 59(3) :341-8
|
|
M15 (galantide)
|
antagonist
|
|
|
Hill coefficient:0.4-0.5
|
Ogren SO, et al. Eur J Pharmacol. 1993;242(1):59-64
|
|
Galnon
|
non-peptide agonist
|
|
spinal cord membranes
|
KD:6+/-0.6 microM
|
Wu WP, et al. Eur J Pharmacol. 2003 ;482(1-3):133-7
|
|
M242
|
|
|
Bowes cells& Chinese hamster ovary cells
|
at hGalR1<1 nM and at hGalR2<10 nM
|
Saar K, et al. Regul Pept. 2001; 102(1) :15-9
|
|
M38, [galanin(1-13)- (Ala-Leu)3-Ala amide]
|
antagonist
|
|
|
|
Xu XJ, et al. Br J Pharmacol. 1995 Oct;116(3):2076-80
|
|
M40, [galanin(1-13) -Pro-Pro-(Ala-Leu)2-Ala amide]
|
antagonist in vivo
|
|
|
|
Xu XJ, et al. Br J Pharmacol. 1995 Oct;116(3):2076-80
|
C7 [galanin (1-13)-spantide] 11 |
antagonist |
|
|
|
Xu XJ, et al. Br J Pharmacol. 1995 Oct;116(3):2076-80
|
|
Galanin (Porcine)
|
agonist
|
[125I]galanin (porcine)
|
Bowes melanoma cell line
|
KD = 0.05 +/- 0.01 nM; Bmax = 135 +/- 7 fmol/mg protein
|
Heuillet E, et al. Eur J Pharmacol. 1994 ; 269 (2):139-47
|
|
Galanin (Human)
|
agonist
|
[125I]galanin (porcine)
|
Bowes melanoma cell line
|
IC50 = 0.35 +/- 0.13 nM
|
Heuillet E, et al. Eur J Pharmacol. 1994 ; 269 (2):139-47
|
|
|
|

|

|

|
|
Mean
values ± S.E. of 6-9 determinations from 2-3 different
experiments (receptor binding) or those of 4 determinations from
2 different experiments (GTPγS binding)
are shown. |
|
|
Receptor
binding (IC50)
|
[35S]GTPγS binding (EC50)
|
GALR1 |
GALR2 |
GALR1
|
GALR2
|
|
|
nM
|
nM
|
|
Rat galanin
|
0.097
± 0.004
|
0.48
± 0.02
|
0.16
± 0.02
|
5.2
± 0.5
|
|
Porcine GALP
|
4.3
± 0.09
|
0.24
± 0.01
|
30
± 4
|
2.4
± 0.4
|
Ohtaki T, et al. J Biol Chem. 1999 Dec 24;274(52):37041-5
|
|
|
EC50,
M of inhibition of adenylate cyclase activity in rat hippocampal
membranes |
KD, M |
Basal |
Forskolin stimulated |
Rat hippocampus |
Bowes cells
(GalR1) |
Galanin |
1.1
× 10-9 |
1.1
× 10-9 |
0.8
× 10-9 |
0.4
× 10-9 |
Galnon |
8
× 10-6 |
10
× 10-6 |
4.8
× 10-6 |
2.9
× 10-6 |
Saar K,
et al. Proc Natl Acad Sci U S A. 2002 May 14;99(10):7136-41

Saturation isotherms of 125I-human galanin to human
GALR1 receptors in HEK293E membranes and whole cells. Saturation
experiments were performed with the addition of 125I-human
galanin up to 5 nM, whereas from 5.0 to 60 nM, unlabeled
cold human galanin was added along with a fixed concentration of
1.5 to 3.0 nM 125I-human galanin as indicated in
"Methods." A, Depicted is a saturation isotherm (and
Scatchard transformation inset) using GALR1 membranes that is
representative of four experiments. Nonlinear regression analysis (LIGAND)
significantly fit (P < .05) a two site/state model better than
a one site/state model. B, A representative saturation isotherm (and
Scatchard transformation inset) using GALR1 whole cells. Depicted are data
that are representative of three independent experiments (KDH = 419 pM,
KDL = 5.8 nM; P = .07, LIGAND).
As reported in "Results" and shown in table 1, a composite
analysis (LIGAND) using data from three experiments (64 individual
data points) was performed to adequately defined the low-affinity site.
This analysis gave sufficient statistical power to yield a significant
two-site/two state model (P < .001) with binding parameters
(see table 1) that closely matched this individual experiment.
Fitzgerald
LW, et al. J Pharmacol Exp Ther. 1998 Nov;287(2):448-56
|
|
|
Membranes
|
|
|
KDH (pM)
|
48.9
± 7.3
|
|
BmaxH (fmol/mg protein)
|
554.3
± 24.9
|
|
% Total
|
21.0
|
|
KDL (nM)
|
14.7
± 4.3
|
|
BmaxL (pmol/mg protein)
|
2.07
± 0.13
|
|
% Total
|
79.0
|
|
Whole cells
|
|
|
KDH (pM)
|
288.0
|
|
BmaxH
|
3,493
receptors/cell, (94 fmol/mg proteina)
|
|
% Total
|
18.8
|
|
KDL (nM)
|
12.8
|
|
BmaxL
|
15,057
receptors/cell, (425 fmol/mg proteina)
|
|
% Total
|
81.2
|
Binding parameters were determined as described in
"Methods." All values for the membrane-based assay represent
the mean ± S.E.M. of three to four experiments all carried
out in duplicate or triplicate. For the whole cell saturation isotherm,
a composite, nonlinear regression analysis of data from three
independent experiments was required to adequately determine the low
affinity component. Data from both membranes and whole cells were best
fit by a two-state/two-site model (P < .05).
Bmax
values from the whole cell saturation isotherm were expressed
conventionally as receptors/cell, but also as fmol/mg protein assuming
a membrane protein yield of 60 ×g/106 GALR1 HEK293E
cells
Fitzgerald
LW, et al. J Pharmacol Exp Ther. 1998 Nov;287(2):448-56

Fitzgerald
LW, et al. J Pharmacol Exp Ther. 1998 Nov;287(2):448-56
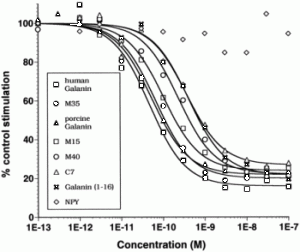
Fitzgerald
LW, et al. J Pharmacol Exp Ther. 1998 Nov;287(2):448-56
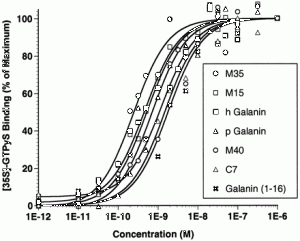
Fitzgerald LW, et al. J Pharmacol Exp Ther. 1998
Nov;287(2):448-56
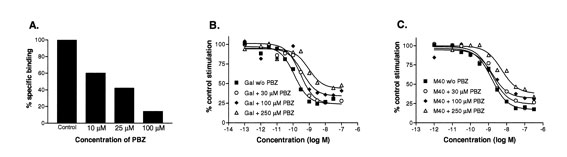
Fitzgerald LW, et al. J Pharmacol Exp Ther. 1998 Nov;287(2):448-56
Galanin Sequence Comprison among Species
Santic et al. Alarin is a vasoactive peptide.
Proc Natl Acad Sci U S A. 2007 Jun 12;104(24):10217-22.
Janovick et al. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target.
J Clin Endocrinol Metab. 2002 Jul;87(7):3255-62.
|
|
|
galp;galpnew
%galanin%
|
|
|


