
Bone remodeling, the function affected in
osteoporosis, the most common of bone diseases, comprises two
phases: bone formation by matrix-producing osteoblasts1 and bone
resorption
by osteoclasts2. The demonstration that the
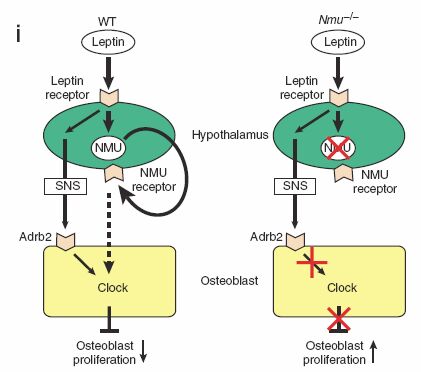
anorexigenic hormone leptin3–5 inhibits bone formation through a
hypothalamic relay6,7 suggests that other molecules that affect
energy metabolism in the hypothalamus could also modulate bone mass.
Neuromedin U (NMU) is an anorexigenic
neuropeptide that acts
independently of leptin through poorly defined mechanisms8,9. Here
we show that Nmu-deficient (Nmu–/–) mice have high bone mass owing
to an increase in bone formation; this is more prominent in male
mice than female mice. Physiological and cell-based assays indicate
that
NMU acts in the central nervous system, rather than directly
on bone cells, to regulate bone remodeling. Notably, leptin- or
sympathetic nervous system–mediated inhibition of bone formation6,7
was abolished in Nmu–/– mice, which show an altered bone expression
of molecular clock genes (mediators of the inhibition of bone
formation by leptin). Moreover, treatment of wild-type mice with a
natural agonist for the NMU receptor decreased bone mass.
Collectively, these results suggest that
NMU may be the first
central mediator of leptin-dependent regulation of bone mass
identified to date. Given the existence of inhibitors and activators
of NMU action10, our results may influence the treatment of diseases
involving low bone mass, such as osteoporosis.
 |
 |
 |
| |
| NMU receptor type-1 (NMU1R) |
NMU receptor type-2 (NMU2R) |
| FM-3/GPR66 |
FM-4/TGR-1 |
| Peripheral tissues |
CNS: PVN & SCN |
| NMU = NMS |
NMS > NMU |
|
|
 |
 |
 |
 |
 |
 |
| |
| Neuromedin U |
Neuromedin S |
| Brain-gut neuropeptide |
Neuro & Immunopeptide |
| Suppression of feeding |
Circadian rhythm regulation in the SCN |
| Regulation of energy homeostasis |
Immune response in the spleen |
| Elevation of arterial blood pressures and control of local blood flow |
Spermatogenesis in the testis |
|
|
 |
 |
 |
Sato S. et al. NATURE MEDICINE, published online 16 September 2007; doi:10.1038/nm1640
Neuromedin U (NMU) is a neuropeptide with potent
activity on smooth muscle which was isolated first from porcine
spinal cord and later from other species. It is widely distributed
in the gut and central nervous system. Peripheral activities of NMU
include stimulation of smooth muscle, increase of blood pressure,
alteration of ion transport in the gut, control of local blood flow
and regulation of adrenocortical function. An NMU receptor has not
been molecularly identified. Here we show that the previously
described orphan G-protein-coupled receptor FM-3 (ref. 15) and a
newly discovered one (FM-4) are cognate receptors for NMU. FM-3,
designated NMU1R, is abundantly expressed in peripheral tissues
whereas FM-4, designated NMU2R, is expressed in specific regions of
the brain. NMU is expressed in the ventromedial hypothalamus in the
rat brain, and its level is significantly reduced following fasting.
Intracerebroventricular administration of NMU markedly suppresses
food intake in rats. These findings provide a molecular basis for
the biochemical activities of NMU and may indicate that NMU is
involved in the central control of feeding (Phoenix's Neuromedin
U and other eighty-three peptides have been used in this
research.)

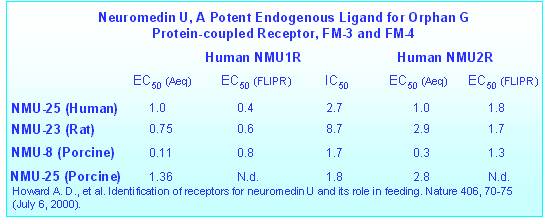
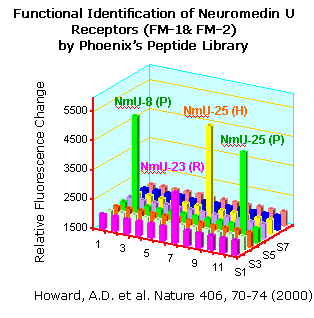
HOWARD,A.D. et al. Identification of Receptors for Neuromedin U
and its Role in Feeding. Nature 406, 70-75 (July 6,
2000).
The structural similarities of NMS and NMU on the gene and proprotein levels were observed among mouse, rat and human,
indicating that the divergence of the two genes encoding these peptides preceded the rodent/primate split during the process of
evolution. In addition, the NMS proprotein contains another novel 34-residue peptide, similar to the novel 33-residue peptide that Lo
et al (1992) speculated to exist in the NMU proprotein. Indeed, we purified such an endogenous 33-residue peptide derived from the NMU
proprotein from rat brain and small intestine extracts. Both synthetic 33- and 34-residue peptides derived from the NMU and NMS
proproteins show potent prolactin-releasing activity upon ICV administration in rats.
The EMBO Journal (2005), 1–11

Expression of Neuromedin U (2.3 KDa) and Prepro-Neuromedin U (18 KDa) in the SD rat brain can be detected with Phoenix's Neuromedin U (Rat) Western Blot Kit (WBK-046-41)Visualization of Neuromedin U Receptors (FM-3 &
FM-4) with FAM labeled Neuromedin U


