| Image | Photomicrographs of sections through rat major pelvic ganglia labeled with CART antisera or CART antisera preabsorbed with the CART peptide 55-102 using the immunoperoxidase method. A) Low-magnification view showing that numerous ganglion cells are strongly labeled. B) Higher-magnification view of an area outlined in A in which CART-LI was detected in some of the smaller-diameter ganglion cells. Some of the larger-diameter ganglion cells, which are not labeled, are invested with varicose CART-LI endings (arrows). C) A section of major pelvic ganglion showing clusters of intensely labeled, small-diameter, CART-positive cells, which are boxed in. D) A section of major pelvic ganglion processed with CART antisera preabsorbed with the peptide (10 µg/ml). Immunoreactivity is not detectable in this section. Bar = 100 µm (A and D), and 25 µm (B), and 50 µm (C) [Dun et al. Biol Reprod. 2000 Nov;63(5):1518-24.] 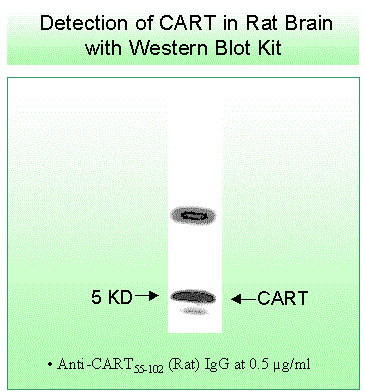 
Protocol for Immunohistochemistry
Rats were anesthetized with urethane (1.2 g/kg i.p.) and intracardially perfused with 0.1 M PBS, followed by freshly prepared, 4% paraformaldehyde in PBS. The epididymis, vas deferens and major pelvic ganglia were removed, postfixed for 2 h, and immersed in 30% sucrose/PBS overnight. Tissues were sectioned to 40 µm with a Vibratome (Technical Products International, Inc., St. Louis, MO) and processed for CART-LI by the avidin-biotin complex (ABC) or fluorescent techniques, as described elsewhere [21, 22]. In addition, some sections were set aside for double-labeling experiments, in which only the fluorescent method was used [21, 22].
In the ABC method, tissues were first treated with 3% H2O2 to quench endogenous peroxidase, washed several times in Tris-buffered saline, and blocked with 10% normal goat sera (Vector Laboratories, Burlingame, CA). Tissues were incubated in the primary antibody to CART peptide fragment 55-102 (1:10 000 dilution with 0.4% Triton X-100 and 1% BSA in PBS) for 48 h at 4°C with gentle agitation. The CART antiserum, a rabbit polyclonal from Phoenix Pharmaceuticals, Inc. (Mountain View, CA), exhibits 100% cross-reactivity with the rat CART peptide 55-102 (Phoenix Pharmaceuticals). After thorough rinsing, sections were incubated with biotinylated antirabbit immunoglobulin (Ig) G (1:150 dilution; Vector Laboratories) for 2 h. Sections were rinsed with PBS and incubated in ABC solution for 1 h (1:100 dilution; Vector Laboratories). After several rinses in Tris-buffered saline, sections were developed in diaminobenzidine-H2O2 solution and washed for at least 2 h with Tris-buffered saline. Sections were mounted on slides with 0.25% gel alcohol, air-dried, dehydrated with absolute alcohol followed by xylene, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA).
For the fluorescent method, tissues were first blocked with 10% normal goat sera and then incubated with CART antisera (1:2 000 dilution with 0.4% Triton X-100 and 1% BSA in PBS) for 48 h in a cold room with gentle agitation. After several washes with PBS, sections were incubated with biotinylated antirabbit IgG (1:50 dilution; Vector Laboratories) for 2 h. After several washes in PBS, tissues were incubated with Fluorescein Avidin D (1:50 dilution; Vector Laboratories). Lastly, tissues were washed for 30 min with PBS, mounted in Citifluor (Ted Pella, Redding, CA), and coverslipped.
In the case of double-labeling studies, the technique of sequential labeling with primary antisera from two different hosts was used . Tissues were first processed for fluorescent CART-LI as described earlier. Thereafter, tissues were washed with PBS for at least 2 h, blocked with normal horse sera, and then incubated with tyrosine hydroxylase (TH) antisera (1:500 dilution with 0.4% Triton X-100 and 1% BSA in PBS) for 48 h in a cold room with gentle agitation. The TH antiserum was a mouse monoclonal from Chemicon International, Inc. (Temecula, CA), and the specificity of the antibody has been extensively evaluated . After washing with PBS for 30 min, tissues were incubated with Avidin Texas Red (Vector Laboratories) for 4 h, washed for 30 min with PBS, mounted in Citifluor, and coverslipped. Sections were examined with a Nikon EC600 fluorescent microscope and photographed. [Dun et al. Biol Reprod. 2000 Nov;63(5):1518-24.] |


