Phoenixin (PNX) is a 14-amino acid amidated peptide (PNX-14) or an N-terminal extended 20-residue amidated peptide (PNX-20) recently identified in neural and non-neural tissue. Mass spectrometry analysis identified a major peak corresponding to PNX-14, with negligible PNX-20, in mouse spinal cord extracts. Using a previously characterized antiserum that recognized both PNX-14 and PNX-20, PNX-immunoreactivity (irPNX) was detected in a population of dorsal root ganglion (DRG) cells and in cell processes densely distributed to the superficial layers of the dorsal horn; irPNX cell processes were also detected in the skin. The retrograde tracer, Fluorogold, injected subcutaneously (s.c.) to the back of the cervical and thoracic spinal cord of mice, labeled a population of DRG, some of which were also irPNX. PNX-14 (2, 4 and 8 mg/kg) injected s.c.to the nape of the neck provoked dose-dependent repetitive scratching bouts directed to the back of the neck with the hindpaws. The number of scratching bouts varied from 16-95 in 30 min, commencing within 5 min post-injection and lasted 10-15 min. Pretreatment of mice at -20 min with nalfurafine (20 μg/kg, s.c.), the kappa opioid receptor agonist, significantly reduced the number of bouts induced by PNX-14 (4 mg/kg) compared with that of saline-pretreated mice. Our results suggest that the peptide, PNX-14, serves as one of the endogenous signal molecules transducing itch sensation in the mouse.
Alan Cowan, Rong-Ming Lyu, Yi-Hung Chen et al., 2015 Sep 25. pii: S0306-4522(15)00882-9. doi: 10.1016/j.neuroscience.2015.09.055. [Epub ahead of print]
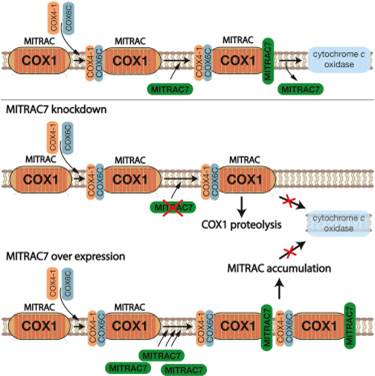
Cytochrome c oxidase, the terminal enzyme of the respiratory chain, is assembled from mitochondria- and nuclear-encoded subunits. The MITRAC complex represents the central assembly intermediate during this process as it receives imported subunits and regulates mitochondrial translation of COX1 mRNA. The molecular processes that promote and regulate the progression of assembly downstream of MITRAC are still unknown. Here, we identify MITRAC7 as a constituent of a late form of MITRAC and as a COX1-specific chaperone. MITRAC7 is required for cytochrome c oxidase biogenesis. Surprisingly, loss of MITRAC7 or an increase in its amount causes selective cytochrome c oxidase deficiency in human cells. We demonstrate that increased MITRAC7 levels stabilize and trap COX1 in MITRAC, blocking progression in the assembly process. In contrast,MITRAC7 deficiency leads to turnover of newly synthesized COX1. Accordingly, MITRAC7 affects the biogenesis pathway by stabilizing newly synthesized COX1 in assembly intermediates, concomitantly preventing turnover.
| 
|
•MITRAC7 is a component of early COX1 assembly intermediates
•Overexpression of MITRAC7 causes accumulation of complex IV assembly intermediates
•Lack of MITRAC7 causes turn over of complex IV assembly intermediates
•A quality control checkpoint exists for COX1 assembly intermediates |
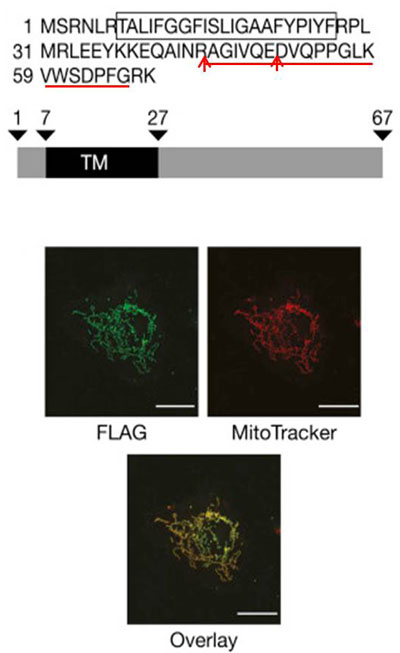
MITRAC7 is the uncharacterized C4ORF52 protein and displays a single predicted transmembrane span, but lacks a predictable N-terminal presequence for translocation across the inner mitochondrial membrane.
To confirm the mitochondrial localization of MITRAC7, we transfected U2OS cells with a MITRAC7FLAG-expressing plasmid. MITRAC7 was detected with monoclonal anti-FLAG antibodies and mitochondrial networks were visualized with MitoTracker red. Diffraction-limited confocal microscopy was performed and superimposition of green and red signals supports the mitochondrial localization of MITRAC7 |
 |
Dennerlein S, Oeljeklaus S, Jans D et al., Cell Rep. 2015 Sep 8;12(10):1644-55. doi: 10.1016/j.celrep.2015.08.009. Epub 2015 Aug 28.


